Di-leucine signals mediate targeting of tyrosinase and synaptotagmin to synaptic-like microvesicles within PC12 cells
- PMID: 10564285
- PMCID: PMC25693
- DOI: 10.1091/mbc.10.11.3979
Di-leucine signals mediate targeting of tyrosinase and synaptotagmin to synaptic-like microvesicles within PC12 cells
Abstract
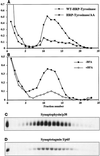
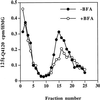
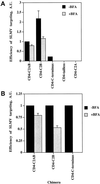
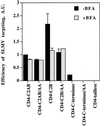
One pathway in forming synaptic-like microvesicles (SLMV) involves direct budding from the plasma membrane, requires adaptor protein 2 (AP2) and is brefeldin A (BFA) resistant. A second route leads from the plasma membrane to an endosomal intermediate from which SLMV bud in a BFA-sensitive, AP3-dependent manner. Because AP3 has been shown to bind to a di-leucine targeting signal in vitro, we have investigated whether this major class of targeting signals is capable of directing protein traffic to SLMV in vivo. We have found that a di-leucine signal within the cytoplasmic tail of human tyrosinase is responsible for the majority of the targeting of HRP-tyrosinase chimeras to SLMV in PC12 cells. Furthermore, we have discovered that a Met-Leu di-hydrophobic motif within the extreme C terminus of synaptotagmin I supports 20% of the SLMV targeting of a CD4-synaptotagmin chimera. All of the traffic to the SLMV mediated by either di-Leu or Met-Leu is BFA sensitive, strongly suggesting a role for AP3 and possibly for an endosomal intermediate in this process. The differential reduction in SLMV targeting for HRP-tyrosinase and CD4-synaptotagmin chimeras by di-alanine substitutions or BFA treatment implies that different proteins use the two routes to the SLMV to differing extents.
Figures



Similar articles
-
Sorting to synaptic-like microvesicles from early and late endosomes requires overlapping but not identical targeting signals.Mol Biol Cell. 2000 May;11(5):1801-14. doi: 10.1091/mbc.11.5.1801. Mol Biol Cell. 2000. PMID: 10793153 Free PMC article.
-
A complex web of signal-dependent trafficking underlies the triorganellar distribution of P-selectin in neuroendocrine PC12 cells.J Cell Biol. 1999 Jun 28;145(7):1419-33. doi: 10.1083/jcb.145.7.1419. J Cell Biol. 1999. PMID: 10385522 Free PMC article.
-
Secretagogue-triggered transfer of membrane proteins from neuroendocrine secretory granules to synaptic-like microvesicles.Mol Biol Cell. 1999 Aug;10(8):2619-30. doi: 10.1091/mbc.10.8.2619. Mol Biol Cell. 1999. PMID: 10436017 Free PMC article.
-
The synaptotagmins: calcium sensors for vesicular trafficking.Neuroscientist. 2004 Dec;10(6):566-74. doi: 10.1177/1073858404268770. Neuroscientist. 2004. PMID: 15534041 Review.
-
Synaptic like microvesicles: do they participate in regulated exocytosis?Neurochem Int. 1995 Sep;27(3):219-26. doi: 10.1016/0197-0186(95)00005-s. Neurochem Int. 1995. PMID: 8520460 Review.
Cited by
-
Sorting of the vesicular GABA transporter to functional vesicle pools by an atypical dileucine-like motif.J Neurosci. 2013 Jun 26;33(26):10634-46. doi: 10.1523/JNEUROSCI.0329-13.2013. J Neurosci. 2013. PMID: 23804087 Free PMC article.
-
BLOC-1 complex deficiency alters the targeting of adaptor protein complex-3 cargoes.Mol Biol Cell. 2006 Sep;17(9):4014-26. doi: 10.1091/mbc.e06-02-0103. Epub 2006 Jun 7. Mol Biol Cell. 2006. PMID: 16760431 Free PMC article.
-
Rab4 regulates formation of synaptic-like microvesicles from early endosomes in PC12 cells.Mol Biol Cell. 2001 Nov;12(11):3703-15. doi: 10.1091/mbc.12.11.3703. Mol Biol Cell. 2001. PMID: 11694600 Free PMC article.
-
Functions of adaptor protein (AP)-3 and AP-1 in tyrosinase sorting from endosomes to melanosomes.Mol Biol Cell. 2005 Nov;16(11):5356-72. doi: 10.1091/mbc.e05-07-0626. Epub 2005 Sep 14. Mol Biol Cell. 2005. PMID: 16162817 Free PMC article.
-
Expression of OA1 limits the fusion of a subset of MVBs with lysosomes - a mechanism potentially involved in the initial biogenesis of melanosomes.J Cell Sci. 2013 Nov 15;126(Pt 22):5143-52. doi: 10.1242/jcs.128561. Epub 2013 Sep 4. J Cell Sci. 2013. PMID: 24006264 Free PMC article.
References
-
- Bauerfeind RA, Regnier-Vigouroux A, Flatmark T, Huttner WB. Selective storage of acetylcholine, but not catecholamines, in neuroendocrine synaptic-like microvesicles of early endosomal origin. Neuron. 1993;11:105–121. - PubMed
-
- Beermann F, Orlow SJ, Boissy RE, Schmidt A, Boissy YL, Lamoreux ML. Misrouting of tyrosinase with a truncated cytoplasmic tail as a result of the murine platinum (Cp) mutation. Exp Eye Res. 1995;61:599–607. - PubMed
-
- Bhatnagar V, Anjaiah S, Puri N, Darshanam BA, Ramaiah A. pH of melanosomes of B16 murine melanoma is acidic: its physiologic importance in the regulation of melanin biosynthesis. Arch Biochem Biophys. 1993;307:183–192. - PubMed
-
- Blackstone CD, Moss SJ, Martin LJ, Levey AI, Price DL, Huganir RL. Biochemical characterization and localization of a non-N-methyl-d-aspartate glutamate receptor in rat brain. J Neurochem. 1992;58:1118–1126. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

