Differential regulation of secretory compartments containing the insulin-responsive glucose transporter 4 in 3T3-L1 adipocytes
- PMID: 10564264
- PMCID: PMC25660
- DOI: 10.1091/mbc.10.11.3675
Differential regulation of secretory compartments containing the insulin-responsive glucose transporter 4 in 3T3-L1 adipocytes
Abstract
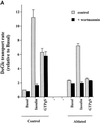
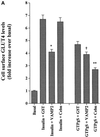
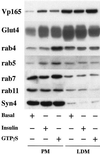
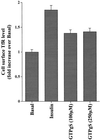
Insulin and guanosine-5'-O-(3-thiotriphosphate) (GTPgammaS) both stimulate glucose transport and translocation of the insulin-responsive glucose transporter 4 (GLUT4) to the plasma membrane in adipocytes. Previous studies suggest that these effects may be mediated by different mechanisms. In this study we have tested the hypothesis that these agonists recruit GLUT4 by distinct trafficking mechanisms, possibly involving mobilization of distinct intracellular compartments. We show that ablation of the endosomal system using transferrin-HRP causes a modest inhibition ( approximately 30%) of insulin-stimulated GLUT4 translocation. In contrast, the GTPgammaS response was significantly attenuated ( approximately 85%) under the same conditions. Introduction of a GST fusion protein encompassing the cytosolic tail of the v-SNARE cellubrevin inhibited GTPgammaS-stimulated GLUT4 translocation by approximately 40% but had no effect on the insulin response. Conversely, a fusion protein encompassing the cytosolic tail of vesicle-associated membrane protein-2 had no significant effect on GTPgammaS-stimulated GLUT4 translocation but inhibited the insulin response by approximately 40%. GTPgammaS- and insulin-stimulated GLUT1 translocation were both partially inhibited by GST-cellubrevin ( approximately 50%) but not by GST-vesicle-associated membrane protein-2. Incubation of streptolysin O-permeabilized 3T3-L1 adipocytes with GTPgammaS caused a marked accumulation of Rab4 and Rab5 at the cell surface, whereas other Rab proteins (Rab7 and Rab11) were unaffected. These data are consistent with the localization of GLUT4 to two distinct intracellular compartments from which it can move to the cell surface independently using distinct sets of trafficking molecules.
Figures





Similar articles
-
Vesicle-associated membrane protein 2 plays a specific role in the insulin-dependent trafficking of the facilitative glucose transporter GLUT4 in 3T3-L1 adipocytes.J Biol Chem. 1998 Jan 16;273(3):1444-52. doi: 10.1074/jbc.273.3.1444. J Biol Chem. 1998. PMID: 9430681
-
Protein kinase B stimulates the translocation of GLUT4 but not GLUT1 or transferrin receptors in 3T3-L1 adipocytes by a pathway involving SNAP-23, synaptobrevin-2, and/or cellubrevin.J Biol Chem. 1999 Oct 1;274(40):28087-95. doi: 10.1074/jbc.274.40.28087. J Biol Chem. 1999. PMID: 10497159
-
Compartment ablation analysis of the insulin-responsive glucose transporter (GLUT4) in 3T3-L1 adipocytes.Biochem J. 1996 Apr 15;315 ( Pt 2)(Pt 2):487-95. doi: 10.1042/bj3150487. Biochem J. 1996. PMID: 8615819 Free PMC article.
-
Role of SNARE's in the GLUT4 translocation response to insulin in adipose cells and muscle.J Basic Clin Physiol Pharmacol. 1998;9(2-4):153-65. doi: 10.1515/jbcpp.1998.9.2-4.153. J Basic Clin Physiol Pharmacol. 1998. PMID: 10212832 Review.
-
Intracellular organization of insulin signaling and GLUT4 translocation.Recent Prog Horm Res. 2001;56:175-93. doi: 10.1210/rp.56.1.175. Recent Prog Horm Res. 2001. PMID: 11237212 Review.
Cited by
-
Reduced insulin-stimulated GLUT4 bioavailability in stroke-prone spontaneously hypertensive rats.Diabetologia. 2005 Mar;48(3):539-46. doi: 10.1007/s00125-005-1674-x. Epub 2005 Feb 24. Diabetologia. 2005. PMID: 15729573
-
GLUT4 retention in adipocytes requires two intracellular insulin-regulated transport steps.Mol Biol Cell. 2002 Jul;13(7):2421-35. doi: 10.1091/mbc.e02-02-0071. Mol Biol Cell. 2002. PMID: 12134080 Free PMC article.
-
Insulin-regulated release from the endosomal recycling compartment is regulated by budding of specialized vesicles.Mol Biol Cell. 2001 Nov;12(11):3489-501. doi: 10.1091/mbc.12.11.3489. Mol Biol Cell. 2001. PMID: 11694583 Free PMC article.
-
Syntaxin 6 regulates Glut4 trafficking in 3T3-L1 adipocytes.Mol Biol Cell. 2003 Jul;14(7):2946-58. doi: 10.1091/mbc.e02-11-0722. Epub 2003 Apr 4. Mol Biol Cell. 2003. PMID: 12857877 Free PMC article.
-
Vesicle-associated Membrane Protein 3 (VAMP3) Mediates Constitutive Trafficking of the Renal Co-transporter NKCC2 in Thick Ascending Limbs: ROLE IN RENAL FUNCTION AND BLOOD PRESSURE.J Biol Chem. 2016 Oct 14;291(42):22063-22073. doi: 10.1074/jbc.M116.735167. Epub 2016 Aug 22. J Biol Chem. 2016. PMID: 27551042 Free PMC article.
References
-
- Baldini G, Hohman R, Charron MJ, Lodish HF. Insulin and nonhydrolyzable GTP analogs induce translocation of GLUT4 to the plasma membrane in a-toxin-permeabilized rat adipose cells J. Biol Chem, 1991;266:4037–4040. - PubMed
-
- Bottger G, Nagelkerken B, van der Sluijs P. Rab4 and Rab7 define distinct nonoverlapping endosomal compartments J. Biol Chem, 1996;271:29191–29197. - PubMed
-
- Calderhead DM, Kitagawa K, Tanner LI, Holman GD, Lienhard GE. Insulin regulation of the two glucose transporters in 3T3–L1 adipocytes J. Biol Chem, 1990;265:13800–13808. - PubMed
-
- Chen D, Elmendorf JS, Olson AL, Li X, Earp HS, Pessin JE. Osmotic shock stimulates GLUT4 translocation in 3T3–L1 adipocytes by a novel tyrosine kinase pathway J. Biol Chem. 1997;272:27401–27410. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

