Subcellular localization of tetanus neurotoxin-insensitive vesicle-associated membrane protein (VAMP)/VAMP7 in neuronal cells: evidence for a novel membrane compartment
- PMID: 10559389
- PMCID: PMC6782963
- DOI: 10.1523/JNEUROSCI.19-22-09803.1999
Subcellular localization of tetanus neurotoxin-insensitive vesicle-associated membrane protein (VAMP)/VAMP7 in neuronal cells: evidence for a novel membrane compartment
Abstract
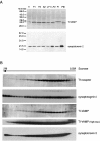
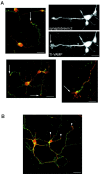
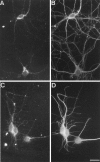
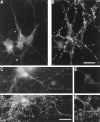
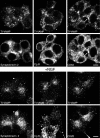
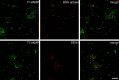
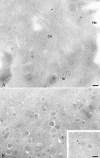
The clostridial neurotoxin-insensitive soluble N-ethylmaleimide-sensitive factor attachment protein (SNAP) receptors, tetanus neurotoxin-insensitive (TI)-vesicle-associated membrane protein (VAMP)/VAMP7, SNAP23, and syntaxin 3 have recently been implicated in transport of exocytotic vesicles to the apical plasma membrane of epithelial cells. This pathway had been shown previously to be insensitive to tetanus neurotoxin and botulinum neurotoxin F. TI-VAMP/VAMP7 is also a good candidate to be implicated in an exocytotic pathway involved in neurite outgrowth because tetanus neurotoxin does not inhibit this process in conditions in which it abolishes neurotransmitter release. We have now found that TI-VAMP/VAMP7 has a widespread distribution in the adult rat brain in which its localization strikingly differs from that of nerve terminal markers. TI-VAMP/VAMP7 does not enrich in synaptic vesicles nor in large dense-core granules but is associated with light membranes. In hippocampal neurons developing in vitro, TI-VAMP/VAMP7 localizes to vesicles in the axonal and dendritic outgrowths and concentrates into the leading edge of the growth cone, a region devoid of synaptobrevin 2, before synaptogenesis. After the onset of synaptogenesis, TI-VAMP/VAMP7 is found predominantly in the somatodendritic domain. In PC12 cells, TI-VAMP/VAMP7 does not colocalize with synaptobrevin 2, chromogranin B, or several markers of endocytic compartments. At the electron microscopic level, TI-VAMP/VAMP7 is mainly associated with tubules and vesicles. Altogether, these results suggest that TI-VAMP/VAMP7 defines a novel membrane compartment in neurite outgrowths and in the somatodendritic domain.
Figures

Similar articles
-
Role of tetanus neurotoxin insensitive vesicle-associated membrane protein (TI-VAMP) in vesicular transport mediating neurite outgrowth.J Cell Biol. 2000 May 15;149(4):889-900. doi: 10.1083/jcb.149.4.889. J Cell Biol. 2000. PMID: 10811829 Free PMC article.
-
A novel tetanus neurotoxin-insensitive vesicle-associated membrane protein in SNARE complexes of the apical plasma membrane of epithelial cells.Mol Biol Cell. 1998 Jun;9(6):1437-48. doi: 10.1091/mbc.9.6.1437. Mol Biol Cell. 1998. PMID: 9614185 Free PMC article.
-
Tetanus neurotoxin-insensitive vesicle-associated membrane protein localizes to a presynaptic membrane compartment in selected terminal subsets of the rat brain.Neuroscience. 2003;122(1):59-75. doi: 10.1016/s0306-4522(03)00567-0. Neuroscience. 2003. PMID: 14596849
-
The tetanus neurotoxin-sensitive and insensitive routes to and from the plasma membrane: fast and slow pathways?Traffic. 2005 May;6(5):366-73. doi: 10.1111/j.1600-0854.2005.00288.x. Traffic. 2005. PMID: 15813747 Review.
-
Multiple roles of the vesicular-SNARE TI-VAMP in post-Golgi and endosomal trafficking.FEBS Lett. 2009 Dec 3;583(23):3817-26. doi: 10.1016/j.febslet.2009.10.026. Epub 2009 Oct 20. FEBS Lett. 2009. PMID: 19837067 Review.
Cited by
-
VAMP7j: A Splice Variant of Human VAMP7 That Modulates Neurite Outgrowth by Regulating L1CAM Transport to the Plasma Membrane.Int J Mol Sci. 2023 Dec 10;24(24):17326. doi: 10.3390/ijms242417326. Int J Mol Sci. 2023. PMID: 38139155 Free PMC article.
-
Regulators of kinesin involved in polarized trafficking and axon outgrowth.J Biol. 2006;5(4):8. doi: 10.1186/jbiol41. Epub 2006 May 25. J Biol. 2006. PMID: 16732897 Free PMC article.
-
pHusion - a robust and versatile toolset for automated detection and analysis of exocytosis.J Cell Sci. 2024 Oct 15;137(20):jcs261828. doi: 10.1242/jcs.261828. Epub 2024 Jun 7. J Cell Sci. 2024. PMID: 38690758 Free PMC article.
-
Vesicle-associated membrane protein 7 is expressed in intestinal ER.J Cell Sci. 2006 Mar 1;119(Pt 5):943-50. doi: 10.1242/jcs.02803. J Cell Sci. 2006. PMID: 16495485 Free PMC article.
-
Myosin Va controls oligodendrocyte morphogenesis and myelination.J Neurosci. 2007 Oct 17;27(42):11366-75. doi: 10.1523/JNEUROSCI.2326-07.2007. J Neurosci. 2007. PMID: 17942731 Free PMC article.
References
-
- Advani RJ, Bae HR, Bock JB, Chao DS, Doung YC, Prekeris R, Yoo JS, Scheller RH. Seven novel mammalian SNARE proteins localize to distinct membrane compartments. J Biol Chem. 1998;273:10317–10324. - PubMed
-
- Banker GA, Cowan WM. Rat hippocampal neurons in dispersed cell culture. Brain Res. 1977;126:397–425. - PubMed
-
- Berditchevski F, Bazzoni G, Hemler ME. Specific association of CD63 with the VLA-3 and VLA-6 integrins. J Biol Chem. 1995;270:17784–17790. - PubMed
-
- Bock JB, Scheller RH. Protein transport—a fusion of new ideas. Nature. 1997;387:133–135. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
