Human immunodeficiency virus replication in a primary effusion lymphoma cell line stimulates lytic-phase replication of Kaposi's sarcoma-associated herpesvirus
- PMID: 10559351
- PMCID: PMC113088
- DOI: 10.1128/JVI.73.12.10329-10338.1999
Human immunodeficiency virus replication in a primary effusion lymphoma cell line stimulates lytic-phase replication of Kaposi's sarcoma-associated herpesvirus
Abstract
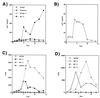
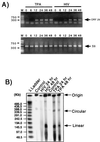
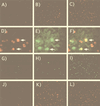
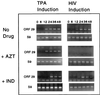
Human immunodeficiency virus (HIV) and Kaposi's sarcoma-associated herpesvirus (KSHV) coinfect many individuals in North America and in parts of Africa. Infection with HIV is a leading risk factor for the development of Kaposi's sarcoma (KS). In this study, we tested the hypothesis that HIV infection of common or adjacent cells would stimulate replication and spread of KSHV. Infection of a primary effusion lymphoma cell line by vesicular stomatitis virus type G-pseudotyped HIV type 1 led to a rapid induction of lytic-phase KSHV replication. Induction of lytic KSHV replication by HIV required active replication of HIV. The addition of the nucleoside reverse transcriptase inhibitor azidothymidine or the protease inhibitor indinavir to the culture prevented HIV spread and inhibited the associated induction of KSHV lytic replication. Lytic replication occurred in both HIV-infected and HIV-uninfected cells within the culture, and could be induced in uninfected cells via a soluble factor released from the HIV-infected cells. Transmission of infectious KSHV to an uninfected target cell was enhanced by HIV replication and was inhibited by antiretroviral drugs. These results may have implications for the pathogenesis and treatment of KS in individuals coinfected with KSHV and HIV.
Figures


Similar articles
-
Human immunodeficiency virus type-1 activates lytic cycle replication of Kaposi's sarcoma-associated herpesvirus through induction of KSHV Rta.Virology. 2002 Jun 5;297(2):270-80. doi: 10.1006/viro.2002.1434. Virology. 2002. PMID: 12083825
-
HIV-1 Vpr Inhibits Kaposi's Sarcoma-Associated Herpesvirus Lytic Replication by Inducing MicroRNA miR-942-5p and Activating NF-κB Signaling.J Virol. 2016 Sep 12;90(19):8739-53. doi: 10.1128/JVI.00797-16. Print 2016 Oct 1. J Virol. 2016. PMID: 27440900 Free PMC article.
-
HIV-1 infection of primary effusion lymphoma cell line triggers Kaposi's sarcoma-associated herpesvirus (KSHV) reactivation.Int J Cancer. 2002 Feb 20;97(6):791-5. doi: 10.1002/ijc.10086. Int J Cancer. 2002. PMID: 11857356
-
[Replication Machinery of Kaposi's Sarcoma-associated Herpesvirus and Drug Discovery Research].Yakugaku Zasshi. 2019;139(1):69-73. doi: 10.1248/yakushi.18-00164-2. Yakugaku Zasshi. 2019. PMID: 30606932 Review. Japanese.
-
Kaposi's sarcoma-associated herpesvirus: the role of lytic replication in targeted therapy.Curr Opin Infect Dis. 2015 Dec;28(6):611-24. doi: 10.1097/QCO.0000000000000213. Curr Opin Infect Dis. 2015. PMID: 26524334 Review.
Cited by
-
GB virus type C E2 protein inhibits human immunodeficiency virus type 1 assembly through interference with HIV-1 gag plasma membrane targeting.J Infect Dis. 2013 Apr;207(7):1171-80. doi: 10.1093/infdis/jit001. Epub 2013 Jan 9. J Infect Dis. 2013. PMID: 23303812 Free PMC article.
-
Characterization of entry mechanisms of human herpesvirus 8 by using an Rta-dependent reporter cell line.J Virol. 2003 Jul;77(14):8147-52. doi: 10.1128/jvi.77.14.8147-8152.2003. J Virol. 2003. PMID: 12829853 Free PMC article.
-
Independent segregation of human immunodeficiency virus type 1 Gag protein complexes and lipid rafts.J Virol. 2003 Feb;77(3):1916-26. doi: 10.1128/jvi.77.3.1916-1926.2003. J Virol. 2003. PMID: 12525626 Free PMC article.
-
Reactivation and Lytic Replication of Kaposi's Sarcoma-Associated Herpesvirus: An Update.Front Microbiol. 2017 Apr 20;8:613. doi: 10.3389/fmicb.2017.00613. eCollection 2017. Front Microbiol. 2017. PMID: 28473805 Free PMC article. Review.
-
Gallic acid inhibits Kaposi's Sarcoma-associated herpesvirus lytic reactivation by suppressing RTA transcriptional activities.Food Sci Nutr. 2020 Dec 3;9(2):847-854. doi: 10.1002/fsn3.2048. eCollection 2021 Feb. Food Sci Nutr. 2020. PMID: 33598168 Free PMC article.
References
-
- Aluigi M G, Albini A, Carlone S, Repetto L, De Marchi R, Icardi A, Moro M, Noonan D, Benelli R. KSHV sequences in biopsies and cultured spindle cells of epidemic, iatrogenic and Mediterranean forms of Kaposi’s sarcoma. Res Virol. 1996;147:267–275. - PubMed
-
- Ambroziak J A, Blackbourn D J, Herndier B G, Glogau R G, Gullett J H, McDonald A R, Lennette E T, Levy J A. Herpes-like sequences in HIV-infected and uninfected Kaposi’s sarcoma patients. Science. 1995;268:582–583. - PubMed
-
- Arvanitakis L, Mesri E A, Nador R G, Said J W, Asch A S, Knowles D M, Cesarman E. Establishment and characterization of a primary effusion (body cavity-based) lymphoma cell line (BC-3) harboring Kaposi’s sarcoma-associated herpesvirus (KSHV/HHV-8) in the absence of Epstein-Barr virus. Blood. 1996;88:2648–2654. - PubMed
-
- Belec L, Tevi-Benissan C, Mohamed A S, Carbonel N, Matta M, Gresenguet G. Enhanced detection of human herpesvirus-8 and cytomegalovirus in semen of HIV-seropositive asymptomatic heterosexual men living in Central Africa. AIDS. 1998;12:674–676. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

