The V protein of simian virus 5 inhibits interferon signalling by targeting STAT1 for proteasome-mediated degradation
- PMID: 10559305
- PMCID: PMC113042
- DOI: 10.1128/JVI.73.12.9928-9933.1999
The V protein of simian virus 5 inhibits interferon signalling by targeting STAT1 for proteasome-mediated degradation
Abstract
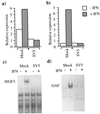
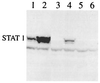
To replicate in vivo, viruses must circumvent cellular antiviral defense mechanisms, including those induced by the interferons (IFNs). Here we demonstrate that simian virus 5 (SV5) blocks IFN signalling in human cells by inhibiting the formation of the IFN-stimulated gene factor 3 and gamma-activated factor transcription complexes that are involved in activating IFN-alpha/beta- and IFN-gamma-responsive genes, respectively. SV5 inhibits the formation of these complexes by specifically targeting STAT1, a component common to both transcription complexes, for proteasome-mediated degradation. Expression of the SV5 structural protein V, in the absence of other virus proteins, also inhibited IFN signalling and induced the degradation of STAT1. Following infection with SV5, STAT1 was degraded in the absence of virus protein synthesis and remained undetectable for up to 4 days postinfection. Furthermore, STAT1 was also degraded in IFN-pretreated cells, even though the cells were in an antiviral state. Since pretreatment of cells with IFN delayed but did not prevent virus replication and protein synthesis, these observations suggest that following infection of IFN-pretreated cells, SV5 remains viable within the cells until they eventually go out of the antiviral state.
Figures
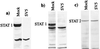
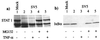
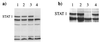
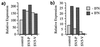
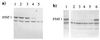

Similar articles
-
Degradation of STAT1 and STAT2 by the V proteins of simian virus 5 and human parainfluenza virus type 2, respectively: consequences for virus replication in the presence of alpha/beta and gamma interferons.J Virol. 2002 Mar;76(5):2159-67. doi: 10.1128/jvi.76.5.2159-2167.2002. J Virol. 2002. PMID: 11836393 Free PMC article.
-
Recovery of paramyxovirus simian virus 5 with a V protein lacking the conserved cysteine-rich domain: the multifunctional V protein blocks both interferon-beta induction and interferon signaling.Virology. 2002 Nov 10;303(1):15-32. doi: 10.1006/viro.2002.1738. Virology. 2002. PMID: 12482655
-
The V protein of human parainfluenza virus 2 antagonizes type I interferon responses by destabilizing signal transducer and activator of transcription 2.Virology. 2001 May 10;283(2):230-9. doi: 10.1006/viro.2001.0856. Virology. 2001. PMID: 11336548
-
New aspects of IFN-alpha/beta signalling in immunity, oncogenesis and bone metabolism.Cancer Sci. 2003 May;94(5):405-11. doi: 10.1111/j.1349-7006.2003.tb01455.x. Cancer Sci. 2003. PMID: 12824884 Free PMC article. Review.
-
Functional Interfaces, Biological Pathways, and Regulations of Interferon-Related DNA Damage Resistance Signature (IRDS) Genes.Biomolecules. 2021 Apr 22;11(5):622. doi: 10.3390/biom11050622. Biomolecules. 2021. PMID: 33922087 Free PMC article. Review.
Cited by
-
Morbillivirus v proteins exhibit multiple mechanisms to block type 1 and type 2 interferon signalling pathways.PLoS One. 2013;8(2):e57063. doi: 10.1371/journal.pone.0057063. Epub 2013 Feb 19. PLoS One. 2013. PMID: 23431397 Free PMC article.
-
The V protein of Tioman virus is incapable of blocking type I interferon signaling in human cells.PLoS One. 2013;8(1):e53881. doi: 10.1371/journal.pone.0053881. Epub 2013 Jan 14. PLoS One. 2013. PMID: 23342031 Free PMC article.
-
A RIG-I 2CARD-MAVS200 Chimeric Protein Reconstitutes IFN-β Induction and Antiviral Response in Models Deficient in Type I IFN Response.J Innate Immun. 2015;7(5):466-81. doi: 10.1159/000375262. Epub 2015 May 5. J Innate Immun. 2015. PMID: 25966783 Free PMC article.
-
Susceptibility of signal transducer and activator of transcription-1-deficient mice to pulmonary fibrogenesis.Am J Pathol. 2005 Nov;167(5):1221-9. doi: 10.1016/S0002-9440(10)61210-2. Am J Pathol. 2005. PMID: 16251407 Free PMC article.
-
Interferon-induced alterations in the pattern of parainfluenza virus 5 transcription and protein synthesis and the induction of virus inclusion bodies.J Virol. 2005 Nov;79(22):14112-21. doi: 10.1128/JVI.79.22.14112-14121.2005. J Virol. 2005. PMID: 16254346 Free PMC article.
References
-
- Boyer S N, Wazer D E, Band V. E7 protein of human papillomavirus-16 induces degradation of retinoblastoma protein through the ubiquitin-proteasome pathway. Cancer Res. 1996;56:4620–4624. - PubMed
-
- Choppin P W. Multiplication of a myxovirus (SV5) with minimal cytopathic effects and without interference. Virology. 1964;23:224–233. - PubMed
-
- Delenda C, Hausmann S, Garcin D, Kolakofsky D. Normal cellular replication of Sendai virus without the trans-frame, nonstructural V protein. Virology. 1997;228:55–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

