The coronin-like protein POD-1 is required for anterior-posterior axis formation and cellular architecture in the nematode caenorhabditis elegans
- PMID: 10557211
- PMCID: PMC317117
- DOI: 10.1101/gad.13.21.2838
The coronin-like protein POD-1 is required for anterior-posterior axis formation and cellular architecture in the nematode caenorhabditis elegans
Abstract
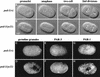
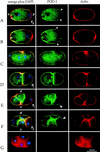
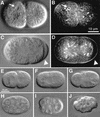
Establishment of anterior-posterior (a-p) polarity in the Caenorhabditis elegans embryo depends on filamentous (F-) actin. Previously, we isolated an F-actin-binding protein that was enriched in the anterior cortex of the one-cell embryo and was hypothesized to link developmental polarity to the actin cytoskeleton. Here, we identify this protein, POD-1, as a new member of the coronin family of actin-binding proteins. We have generated a deletion within the pod-1 gene. Elimination of POD-1 from early embryos results in a loss of physical and molecular asymmetries along the a-p axis. For example, PAR-1 and PAR-3, which themselves are polarized and required for a-p polarity, are delocalized in pod-1 mutant embryos. However, unlike loss of PAR proteins, loss of POD-1 gives rise to the formation of abnormal cellular structures, namely large vesicles of endocytic origin, membrane protrusions, unstable cell divisions, a defective eggshell, and deposition of extracellular material. We conclude that, analogous to coronin, POD-1 plays an important role in intracellular trafficking and organizing specific aspects of the actin cytoskeleton. We propose models to explain how the role of POD-1 in basic cellular processes could be linked to the generation of polarity along the embryonic a-p axis.
Figures


Similar articles
-
Pod-2, along with pod-1, defines a new class of genes required for polarity in the early Caenorhabditis elegans embryo.Dev Biol. 2001 May 15;233(2):412-24. doi: 10.1006/dbio.2001.0234. Dev Biol. 2001. PMID: 11336504
-
CDC-42 regulates PAR protein localization and function to control cellular and embryonic polarity in C. elegans.Curr Biol. 2001 Apr 3;11(7):474-81. doi: 10.1016/s0960-9822(01)00141-5. Curr Biol. 2001. PMID: 11412996
-
Involvement of fatty acid pathways and cortical interaction of the pronuclear complex in Caenorhabditis elegans embryonic polarity.BMC Dev Biol. 2003 Oct 3;3:8. doi: 10.1186/1471-213X-3-8. BMC Dev Biol. 2003. PMID: 14527340 Free PMC article.
-
Establishment of initial asymmetry in early Caenorhabditis elegans embryos.Curr Opin Genet Dev. 1994 Aug;4(4):563-8. doi: 10.1016/0959-437x(94)90073-c. Curr Opin Genet Dev. 1994. PMID: 7950325 Review.
-
Intercellular junctions and cellular polarity: the PAR-aPKC complex, a conserved core cassette playing fundamental roles in cell polarity.Curr Opin Cell Biol. 2001 Oct;13(5):641-8. doi: 10.1016/s0955-0674(00)00264-7. Curr Opin Cell Biol. 2001. PMID: 11544035 Review.
Cited by
-
A genomewide survey of developmentally relevant genes in Ciona intestinalis. VII. Molecules involved in the regulation of cell polarity and actin dynamics.Dev Genes Evol. 2003 Jun;213(5-6):273-83. doi: 10.1007/s00427-003-0325-9. Epub 2003 May 10. Dev Genes Evol. 2003. PMID: 12740699
-
Mechanochemical Control of Symmetry Breaking in the Caenorhabditis elegans Zygote.Front Cell Dev Biol. 2021 Jan 18;8:619869. doi: 10.3389/fcell.2020.619869. eCollection 2020. Front Cell Dev Biol. 2021. PMID: 33537308 Free PMC article. Review.
-
Cortical granule exocytosis in C. elegans is regulated by cell cycle components including separase.Development. 2007 Nov;134(21):3837-48. doi: 10.1242/dev.011361. Epub 2007 Oct 3. Development. 2007. PMID: 17913784 Free PMC article.
-
Diverse roles of actin in C. elegans early embryogenesis.BMC Dev Biol. 2007 Dec 24;7:142. doi: 10.1186/1471-213X-7-142. BMC Dev Biol. 2007. PMID: 18157918 Free PMC article.
-
Chondroitin 4-O-Sulfotransferase Is Indispensable for Sulfation of Chondroitin and Plays an Important Role in Maintaining Normal Life Span and Oxidative Stress Responses in Nematodes.J Biol Chem. 2016 Oct 28;291(44):23294-23304. doi: 10.1074/jbc.M116.757328. Epub 2016 Sep 19. J Biol Chem. 2016. PMID: 27645998 Free PMC article.
References
-
- Boehm H, Brinkmann V, Drab M, Henske A, Kurzchalia TV. Mammalian homologues of C. elegans PAR-1 are asymmetrically localized in epithelial cells and may influence their polarity. Curr Biol. 1997;7:603–606. - PubMed
-
- Bowerman B. Maternal control of pattern formation in early Caenorhabditis elegans embryos. In: Pedersen RA, Schatten GP, editors. Current Topics in Developmental Biology. San Diego, CA: Academic Press, Inc.; 1998. pp. 73–117. - PubMed
-
- Boyd L, Guo S, Levitan D, Stinchcomb DT, Kemphues KJ. PAR-2 is asymmetrically distributed and promotes association of P granules and PAR-1 with the cortex in C. elegans embryos. Development. 1996;122:3075–3084. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
