Induction of lung fibrosis in the mouse by intratracheal instillation of fluorescein isothiocyanate is not T-cell-dependent
- PMID: 10550334
- PMCID: PMC1866962
- DOI: 10.1016/S0002-9440(10)65493-4
Induction of lung fibrosis in the mouse by intratracheal instillation of fluorescein isothiocyanate is not T-cell-dependent
Abstract
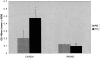
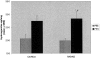
Pulmonary fibrosis is the pathological result of a diverse group of insults. Common features of this group of diseases include chronic inflammation and immune cell activation. The pathogenesis of pulmonary fibrosis is not well defined and the prognosis is poor, highlighting the need for good animal models to elucidate the cellular and molecular events that lead to pulmonary fibrosis. This paper provides insight on a newly described model of pulmonary fibrosis using a single intratracheal challenge with fluorescein isothiocyanate (FITC). Balb-c and C57BL6 mice given intratracheal FITC develop acute lung injury followed by chronic inflammation. Significant increases in lung collagen content compared to saline-treated mice are noted at day 21 after inoculation. T-cell-deficient animals develop similar increases in lung collagen content compared to immunocompetent controls despite the abrogation of specific anti-FITC serum antibodies. Thus, the induction of fibrosis in FITC-challenged mice is not dependent on T cell immunity. Persistent chronic inflammation and acute lung injury may be the inciting events for the development of lung fibrosis in this model.
Figures







Similar articles
-
Protection from fluorescein isothiocyanate-induced fibrosis in IL-13-deficient, but not IL-4-deficient, mice results from impaired collagen synthesis by fibroblasts.J Immunol. 2004 Apr 1;172(7):4068-76. doi: 10.4049/jimmunol.172.7.4068. J Immunol. 2004. PMID: 15034018
-
A novel model for human interstitial lung disease: hapten-driven lung fibrosis in rodents.J Pathol. 1995 Jul;176(3):309-18. doi: 10.1002/path.1711760313. J Pathol. 1995. PMID: 7674093
-
FITC-induced murine pulmonary inflammation: CC10 up-regulation and concurrent Shh expression.Cell Biol Int. 2005 Oct;29(10):868-76. doi: 10.1016/j.cellbi.2005.07.002. Cell Biol Int. 2005. PMID: 16150617
-
Animal models of pulmonary fibrosis.Methods Mol Med. 2005;117:251-9. doi: 10.1385/1-59259-940-0:251. Methods Mol Med. 2005. PMID: 16118457
-
Roles of T lymphocytes in pulmonary fibrosis.J Leukoc Biol. 2008 Feb;83(2):237-44. doi: 10.1189/jlb.0707504. Epub 2007 Oct 25. J Leukoc Biol. 2008. PMID: 17962367 Review.
Cited by
-
The contribution of animal models to understanding the role of the immune system in human idiopathic pulmonary fibrosis.Clin Transl Immunology. 2020 Jul 27;9(7):e1153. doi: 10.1002/cti2.1153. eCollection 2020. Clin Transl Immunology. 2020. PMID: 32742653 Free PMC article.
-
PPARs in alveolar macrophage biology.PPAR Res. 2007;2007:23812. doi: 10.1155/2007/23812. PPAR Res. 2007. PMID: 18000531 Free PMC article.
-
Curcumin inhibits paraquat induced lung inflammation and fibrosis by extracellular matrix modifications in mouse model.Inflammopharmacology. 2016 Dec;24(6):335-345. doi: 10.1007/s10787-016-0286-z. Epub 2016 Oct 20. Inflammopharmacology. 2016. PMID: 27766504
-
Partial characterization of the Sox2+ cell population in an adult murine model of digit amputation.Tissue Eng Part A. 2012 Jul;18(13-14):1454-63. doi: 10.1089/ten.TEA.2011.0550. Epub 2012 Jun 5. Tissue Eng Part A. 2012. PMID: 22530556 Free PMC article.
-
Mesenchymal Stromal Cells for the Treatment of Interstitial Lung Disease in Children: A Look from Pediatric and Pediatric Surgeon Viewpoints.Cells. 2021 Nov 23;10(12):3270. doi: 10.3390/cells10123270. Cells. 2021. PMID: 34943779 Free PMC article. Review.
References
-
- Kimura R, Hu H, Stein-Streilein J: Tolerance to hapten prevents specific delayed type hypersensitivity and pulmonary interstitial fibrosis in the mouse model. Chest 1993 (suppl), 103:122–124S - PubMed
-
- Hu H, Stein-Streilein J: Hapten-immune pulmonary interstitial fibrosis (HIPIF) in mice requires both CD4+ and CD8+ T lymphocytes. J Leukoc Biol 1993, 54:414-422 - PubMed
-
- Roberts SN, Howie SE, Wallace WA, Brown DM, Lamb D, Ramage EA, Donaldson K: A novel model for human interstitial lung disease: hapten-driven lung fibrosis in rodents. J Pathol 1995, 176:309-318 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

