Microglial and astrocyte chemokines regulate monocyte migration through the blood-brain barrier in human immunodeficiency virus-1 encephalitis
- PMID: 10550317
- PMCID: PMC1866982
- DOI: 10.1016/S0002-9440(10)65476-4
Microglial and astrocyte chemokines regulate monocyte migration through the blood-brain barrier in human immunodeficiency virus-1 encephalitis
Abstract
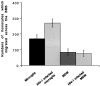
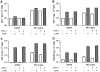
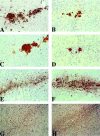
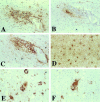
The numbers of immune-activated brain mononuclear phagocytes (MPs) affect the progression of human immunodeficiency virus (HIV)-1-associated dementia (HAD). Such MPs originate, in measure, from a pool of circulating monocytes. To address the mechanism(s) for monocyte penetration across the blood-brain barrier (BBB), we performed cross-validating laboratory, animal model, and human brain tissue investigations into HAD pathogenesis. First, an artificial BBB was constructed in which human brain microvascular endothelial and glial cells-astrocytes, microglia, and/or monocyte-derived macrophages (MDM)-were placed on opposite sides of a matrix-coated porous membrane. Second, a SCID mouse model of HIV-1 encephalitis (HIVE) was used to determine in vivo monocyte blood-to-brain migration. Third, immunohistochemical analyses of human HIVE tissue defined the relationships between astrogliosis, activation of microglia, virus infection, monocyte brain infiltration, and beta-chemokine expression. The results, taken together, showed that HIV-1-infected microglia increased monocyte migration through an artificial BBB 2 to 3.5 times more than replicate numbers of MDM. In the HIVE SCID mice, a marked accumulation of murine MDM was found in areas surrounding virus-infected human microglia but not MDM. For human HIVE, microglial activation and virus infection correlated with astrogliosis, monocyte transendothelial migration, and beta-chemokine expression. Pure cultures of virus-infected and activated microglia or astrocytes exposed to microglial conditioned media produced significant quantities of beta-chemokines. We conclude that microglial activation alone and/or through its interactions with astrocytes induces beta-chemokine-mediated monocyte migration in HAD.
Figures

Similar articles
-
Development of laboratory and animal model systems for HIV-1 encephalitis and its associated dementia.J Leukoc Biol. 1997 Jul;62(1):100-6. doi: 10.1002/jlb.62.1.100. J Leukoc Biol. 1997. PMID: 9226000
-
Human immunodeficiency virus encephalitis in SCID mice.Am J Pathol. 1996 Sep;149(3):1027-53. Am J Pathol. 1996. PMID: 8780406 Free PMC article.
-
Mononuclear phagocytes mediate blood-brain barrier compromise and neuronal injury during HIV-1-associated dementia.J Leukoc Biol. 2000 Sep;68(3):413-22. J Leukoc Biol. 2000. PMID: 10985259 Review.
-
Crosstalk between components of the blood brain barrier and cells of the CNS in microglial activation in AIDS.Brain Pathol. 2001 Jul;11(3):306-12. doi: 10.1111/j.1750-3639.2001.tb00401.x. Brain Pathol. 2001. PMID: 11414473 Free PMC article. Review.
-
A model for monocyte migration through the blood-brain barrier during HIV-1 encephalitis.J Immunol. 1997 Apr 1;158(7):3499-510. J Immunol. 1997. PMID: 9120312
Cited by
-
Reactive astrocytes transduce inflammation in a blood-brain barrier model through a TNF-STAT3 signaling axis and secretion of alpha 1-antichymotrypsin.Nat Commun. 2022 Nov 2;13(1):6581. doi: 10.1038/s41467-022-34412-4. Nat Commun. 2022. PMID: 36323693 Free PMC article.
-
Cross-Talk and Subset Control of Microglia and Associated Myeloid Cells in Neurological Disorders.Cells. 2022 Oct 25;11(21):3364. doi: 10.3390/cells11213364. Cells. 2022. PMID: 36359758 Free PMC article. Review.
-
Cell-type specific differences in antiretroviral penetration and the effects of HIV-1 Tat and morphine among primary human brain endothelial cells, astrocytes, pericytes, and microglia.Neurosci Lett. 2019 Nov 1;712:134475. doi: 10.1016/j.neulet.2019.134475. Epub 2019 Sep 3. Neurosci Lett. 2019. PMID: 31491466 Free PMC article.
-
Rodent model systems for studies of HIV-1 associated dementia.Neurotox Res. 2005 Oct;8(1-2):91-106. doi: 10.1007/BF03033822. Neurotox Res. 2005. PMID: 16260388 Review.
-
Tissue inhibitor of metalloproteinase (TIMP)-1: the TIMPed balance of matrix metalloproteinases in the central nervous system.J Neurosci Res. 2003 Dec 15;74(6):801-6. doi: 10.1002/jnr.10835. J Neurosci Res. 2003. PMID: 14648584 Free PMC article. Review.
References
-
- Navia BA, Jordan BD, Price RW: The AIDS dementia complex. I. Clinical features. Ann Neurol 1986, 19:517-524 - PubMed
-
- Price R, Brew B, Sidtis J, Rosenblum M, Scheck A, Cleary P: The brain and AIDS: central nervous system HIV-1 infection and AIDS dementia complex. Science 1988, 239:586-592 - PubMed
-
- Gendelman HE, Persidsky Y, Ghorpade A, Limoges J, Stins M, Fiala M, Morrisett R: The neuropathogenesis of HIV-1 dementia. AIDS 1997, 11(suppl. A):S35-S45 - PubMed
-
- Sharer LR, Cho E, Epstein LG: Multinucleated giant cells and HTLV-III in AIDS encephalopathy. Hum Pathol 1985, 16:170-176 - PubMed
-
- Budka H: Multinucleated giant cells in the brain: a hallmark of the acquired immunodeficiency syndrome (AIDS). Acta Neuropathol (Berl) 1986, 69:253-256 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

