Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73
- PMID: 10545503
- PMCID: PMC2151184
- DOI: 10.1083/jcb.147.3.599
Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73
Abstract
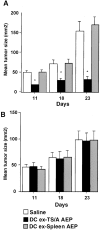
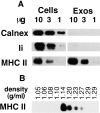
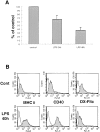
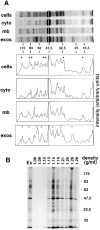
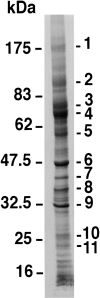
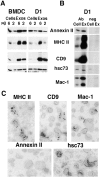
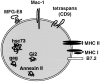
Exosomes are membrane vesicles secreted by hematopoietic cells upon fusion of late multivesicular endosomes with the plasma membrane. Dendritic cell (DC)-derived exosomes induce potent antitumor immune responses in mice, resulting in the regression of established tumors (Zitvogel, L., A. Regnault, A. Lozier, J. Wolfers, C. Flament, D. Tenza, P. Ricciardi-Castagnoli, G. Raposo, and S. Amigorena. 1998. Nat. Med. 4:594-600). To unravel the molecular basis of exosome-induced immune stimulation, we now analyze the regulation of their production during DC maturation and characterize extensively their protein composition by peptide mass mapping. Exosomes contain several cytosolic proteins (including annexin II, heat shock cognate protein hsc73, and heteromeric G protein Gi2alpha), as well as different integral or peripherally associated membrane proteins (major histocompatibility complex class II, Mac-1 integrin, CD9, milk fat globule-EGF-factor VIII [MFG-E8]). MFG-E8, the major exosomal component, binds integrins expressed by DCs and macrophages, suggesting that it may be involved in exosome targeting to these professional antigen-presenting cells. Another exosome component is hsc73, a cytosolic heat shock protein (hsp) also present in DC endocytic compartments. hsc73 was shown to induce antitumor immune responses in vivo, and therefore could be involved in the exosome's potent antitumor effects. Finally, exosome production is downregulated upon DC maturation, indicating that in vivo, exosomes are produced by immature DCs in peripheral tissues. Thus, DC-derived exosomes accumulate a defined subset of cellular proteins reflecting their endosomal biogenesis and accounting for their biological function.
Figures

Similar articles
-
Accumulation of MFG-E8/lactadherin on exosomes from immature dendritic cells.Blood Cells Mol Dis. 2005 Sep-Oct;35(2):81-8. doi: 10.1016/j.bcmd.2005.05.001. Blood Cells Mol Dis. 2005. PMID: 15982908
-
Mature dendritic cells secrete exosomes with strong ability to induce antigen-specific effector immune responses.Blood Cells Mol Dis. 2005 Sep-Oct;35(2):89-93. doi: 10.1016/j.bcmd.2005.05.003. Blood Cells Mol Dis. 2005. PMID: 15990342
-
ICAM-1 on exosomes from mature dendritic cells is critical for efficient naive T-cell priming.Blood. 2005 Jul 1;106(1):216-23. doi: 10.1182/blood-2005-01-0220. Epub 2005 Mar 24. Blood. 2005. PMID: 15790784
-
[Exosomes derived from dendritic cells].J Soc Biol. 2001;195(1):25-7. J Soc Biol. 2001. PMID: 11530496 Review. French.
-
Compartmentalization of class II antigen presentation: contribution of cytoplasmic and endosomal processing.Immunol Rev. 2005 Oct;207:206-17. doi: 10.1111/j.0105-2896.2005.00297.x. Immunol Rev. 2005. PMID: 16181338 Review.
Cited by
-
Trial watch: Dendritic cell-based anticancer therapy.Oncoimmunology. 2014 Dec 21;3(11):e963424. doi: 10.4161/21624011.2014.963424. eCollection 2014 Nov. Oncoimmunology. 2014. PMID: 25941593 Free PMC article. Review.
-
Altered microRNA profiles in bronchoalveolar lavage fluid exosomes in asthmatic patients.J Allergy Clin Immunol. 2013 Mar;131(3):894-903. doi: 10.1016/j.jaci.2012.11.039. Epub 2013 Jan 16. J Allergy Clin Immunol. 2013. PMID: 23333113 Free PMC article.
-
Transplant tolerance: new insights and strategies for long-term allograft acceptance.Clin Dev Immunol. 2013;2013:210506. doi: 10.1155/2013/210506. Epub 2013 May 12. Clin Dev Immunol. 2013. PMID: 23762087 Free PMC article. Review.
-
Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies.J Neurooncol. 2013 May;113(1):1-11. doi: 10.1007/s11060-013-1084-8. Epub 2013 Mar 2. J Neurooncol. 2013. PMID: 23456661 Free PMC article. Review.
-
Reorganization of multivesicular bodies regulates MHC class II antigen presentation by dendritic cells.J Cell Biol. 2001 Oct 1;155(1):53-63. doi: 10.1083/jcb.200103071. J Cell Biol. 2001. PMID: 11581285 Free PMC article.
References
-
- Albert M.L., Sauter B., Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. - PubMed
-
- Amigorena S., Drake J.R., Webster P., Mellman I. Transient accumulation of new class II MHC molecules in a novel endocytic compartment in B lymphocytes. Nature. 1994;369:113–120. - PubMed
-
- Andersen M.H., Berglund L., Rasmussen J.T., Petersen T.E. Bovine PAS-6/7 binds alpha v beta 5 integrins and anionic phospholipids through two domains. Biochemistry. 1997;36:5441–5446. - PubMed
-
- Aoki N., Kishi M., Taniguchi Y., Adachi T., Nakamura R., Matsuda T. Molecular cloning of glycoprotein antigens MGP57/53 recognized by monoclonal antibodies raised against bovine milk fat globule membrane. Biochim. Biophys. Acta. 1995;1245:385–391. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

