Leukocyte recruitment in the cerebrospinal fluid of mice with experimental meningitis is inhibited by an antibody to junctional adhesion molecule (JAM)
- PMID: 10544206
- PMCID: PMC2195675
- DOI: 10.1084/jem.190.9.1351
Leukocyte recruitment in the cerebrospinal fluid of mice with experimental meningitis is inhibited by an antibody to junctional adhesion molecule (JAM)
Abstract
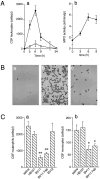
The mechanisms that govern leukocyte transmigration through the endothelium are not yet fully defined. Junctional adhesion molecule (JAM) is a newly cloned member of the immunoglobulin superfamily which is selectively concentrated at tight junctions of endothelial and epithelial cells. A blocking monoclonal antibody (BV11 mAb) directed to JAM was able to inhibit monocyte transmigration through endothelial cells in in vitro and in vivo chemotaxis assays. In this study, we report that BV11 administration was able to attenuate cytokine-induced meningitis in mice. The intravenous injection of BV11 mAb significantly inhibited leukocyte accumulation in the cerebrospinal fluid and infiltration in the brain parenchyma. Blood-brain barrier permeability was also reduced by the mAb. We conclude that JAM may be a new target in limiting the inflammatory response that accompanies meningitis.
Figures
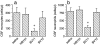



Similar articles
-
Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration.J Cell Biol. 1998 Jul 13;142(1):117-27. doi: 10.1083/jcb.142.1.117. J Cell Biol. 1998. PMID: 9660867 Free PMC article.
-
Reduced expression of junctional adhesion molecule and platelet/endothelial cell adhesion molecule-1 (CD31) at human vascular endothelial junctions by cytokines tumor necrosis factor-alpha plus interferon-gamma Does not reduce leukocyte transmigration under flow.Am J Pathol. 2001 Dec;159(6):2281-91. doi: 10.1016/s0002-9440(10)63078-7. Am J Pathol. 2001. PMID: 11733377 Free PMC article.
-
Neutrophil transmigration under shear flow conditions in vitro is junctional adhesion molecule-C independent.J Immunol. 2007 May 1;178(9):5879-87. doi: 10.4049/jimmunol.178.9.5879. J Immunol. 2007. PMID: 17442972
-
Leukocyte-endothelial-cell interactions in leukocyte transmigration and the inflammatory response.Trends Immunol. 2003 Jun;24(6):327-34. doi: 10.1016/s1471-4906(03)00117-0. Trends Immunol. 2003. PMID: 12810109 Review.
-
The role of JAM-A and PECAM-1 in modulating leukocyte infiltration in inflamed and ischemic tissues.J Leukoc Biol. 2006 Oct;80(4):714-8. doi: 10.1189/jlb.1105645. Epub 2006 Jul 20. J Leukoc Biol. 2006. PMID: 16857733 Review.
Cited by
-
Breaking into the epithelial apical-junctional complex--news from pathogen hackers.Curr Opin Cell Biol. 2004 Feb;16(1):86-93. doi: 10.1016/j.ceb.2003.12.002. Curr Opin Cell Biol. 2004. PMID: 15037310 Free PMC article. Review.
-
Nanowire array chips for molecular typing of rare trafficking leukocytes with application to neurodegenerative pathology.Nanoscale. 2014 Jun 21;6(12):6537-50. doi: 10.1039/c3nr06465d. Nanoscale. 2014. PMID: 24705924 Free PMC article.
-
Buprenorphine decreases the CCL2-mediated chemotactic response of monocytes.J Immunol. 2015 Apr 1;194(7):3246-58. doi: 10.4049/jimmunol.1302647. Epub 2015 Feb 25. J Immunol. 2015. PMID: 25716997 Free PMC article.
-
Elevated levels of homocysteine compromise blood-brain barrier integrity in mice.Blood. 2006 Jan 15;107(2):591-3. doi: 10.1182/blood-2005-06-2506. Epub 2005 Sep 27. Blood. 2006. PMID: 16189268 Free PMC article.
-
Blood-brain barrier: structural components and function under physiologic and pathologic conditions.J Neuroimmune Pharmacol. 2006 Sep;1(3):223-36. doi: 10.1007/s11481-006-9025-3. Epub 2006 Jul 6. J Neuroimmune Pharmacol. 2006. PMID: 18040800 Review.
References
-
- Rowland L.P., Fink M.E., Rubin L. Cerebrospinal fluidblood-brain barrier, brain edema, and hydrocephalus. In: Kandel E.R., Schwartz J., Jessel T.M., editors. Principles of Neural Science. Elsevier Science Inc; New York: 1991. pp. 1050–1060.
-
- Perry V.H., Anthony D.C., Bolton S.J., Brown H.C. The blood-brain barrier and the inflammatory response. Mol. Med. Today. 1997;3:335–341. - PubMed
-
- Quagliarello V.J., Scheld W.M. New perspectives on bacterial meningitis Clin. Infect. Dis. 17 1993. 603 608quiz 609–610 - PubMed
-
- Sharief M.K., Ciardi M., Thompson E.J. Blood-brain barrier damage in patients with bacterial meningitisassociation with tumor necrosis factor-alpha but not interleukin-1 beta. J. Infect. Dis. 1992;166:350–358. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

