Crystal structure of a multifunctional 2-Cys peroxiredoxin heme-binding protein 23 kDa/proliferation-associated gene product
- PMID: 10535922
- PMCID: PMC22917
- DOI: 10.1073/pnas.96.22.12333
Crystal structure of a multifunctional 2-Cys peroxiredoxin heme-binding protein 23 kDa/proliferation-associated gene product
Abstract
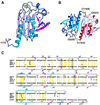
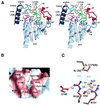
Heme-binding protein 23 kDa (HBP23), a rat isoform of human proliferation-associated gene product (PAG), is a member of the peroxiredoxin family of peroxidases, having two conserved cysteine residues. Recent biochemical studies have shown that HBP23/PAG is an oxidative stress-induced and proliferation-coupled multifunctional protein that exhibits specific bindings to c-Abl protein tyrosine kinase and heme, as well as a peroxidase activity. A 2.6-A resolution crystal structure of rat HBP23 in oxidized form revealed an unusual dimer structure in which the active residue Cys-52 forms a disulfide bond with conserved Cys-173 from another subunit by C-terminal tail swapping. The active site is largely hydrophobic with partially exposed Cys-173, suggesting a reduction mechanism of oxidized HBP23 by thioredoxin. Thus, the unusual cysteine disulfide bond is involved in peroxidation catalysis by using thioredoxin as the source of reducing equivalents. The structure also provides a clue to possible interaction surfaces for c-Abl and heme. Several significant structural differences have been found from a 1-Cys peroxiredoxin, ORF6, which lacks the C-terminal conserved cysteine corresponding to Cys-173 of HBP23.
Figures
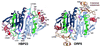
Similar articles
-
Dimer-oligomer interconversion of wild-type and mutant rat 2-Cys peroxiredoxin: disulfide formation at dimer-dimer interfaces is not essential for decamerization.J Biol Chem. 2008 Jan 4;283(1):284-293. doi: 10.1074/jbc.M705753200. Epub 2007 Nov 1. J Biol Chem. 2008. PMID: 17974571
-
Crystal structure of human peroxiredoxin 5, a novel type of mammalian peroxiredoxin at 1.5 A resolution.J Mol Biol. 2001 Aug 24;311(4):751-9. doi: 10.1006/jmbi.2001.4853. J Mol Biol. 2001. PMID: 11518528
-
Crystal structure of an archaeal peroxiredoxin from the aerobic hyperthermophilic crenarchaeon Aeropyrum pernix K1.J Mol Biol. 2005 Nov 25;354(2):317-29. doi: 10.1016/j.jmb.2005.09.006. Epub 2005 Sep 22. J Mol Biol. 2005. PMID: 16214169
-
Bacterial defenses against oxidants: mechanistic features of cysteine-based peroxidases and their flavoprotein reductases.Arch Biochem Biophys. 2005 Jan 1;433(1):240-54. doi: 10.1016/j.abb.2004.09.006. Arch Biochem Biophys. 2005. PMID: 15581580 Review.
-
Thioredoxin networks in the malarial parasite Plasmodium falciparum.Antioxid Redox Signal. 2006 Jul-Aug;8(7-8):1227-39. doi: 10.1089/ars.2006.8.1227. Antioxid Redox Signal. 2006. PMID: 16910770 Review.
Cited by
-
Biomphalaria glabrata peroxiredoxin: effect of schistosoma mansoni infection on differential gene regulation.Mol Biochem Parasitol. 2009 Sep;167(1):20-31. doi: 10.1016/j.molbiopara.2009.04.002. Epub 2009 Apr 11. Mol Biochem Parasitol. 2009. PMID: 19439374 Free PMC article.
-
Redox Regulation of PTEN by Peroxiredoxins.Antioxidants (Basel). 2021 Feb 16;10(2):302. doi: 10.3390/antiox10020302. Antioxidants (Basel). 2021. PMID: 33669370 Free PMC article. Review.
-
Peroxiredoxin functions as a peroxidase and a regulator and sensor of local peroxides.J Biol Chem. 2012 Feb 10;287(7):4403-10. doi: 10.1074/jbc.R111.283432. Epub 2011 Dec 6. J Biol Chem. 2012. PMID: 22147704 Free PMC article. Review.
-
Crystallization and preliminary X-ray diffraction analysis of thioredoxin peroxidase from the aerobic hyperthermophilic archaeon Aeropyrum pernix K1.Acta Crystallogr Sect F Struct Biol Cryst Commun. 2005 Mar 1;61(Pt 3):323-5. doi: 10.1107/S1744309105005294. Epub 2005 Feb 24. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2005. PMID: 16511031 Free PMC article.
-
Crystal structure of the quorum-sensing protein LuxS reveals a catalytic metal site.Proc Natl Acad Sci U S A. 2001 Sep 25;98(20):11169-74. doi: 10.1073/pnas.191223098. Epub 2001 Sep 11. Proc Natl Acad Sci U S A. 2001. PMID: 11553770 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

