Synaptic basis of cortical persistent activity: the importance of NMDA receptors to working memory
- PMID: 10531461
- PMCID: PMC6782911
- DOI: 10.1523/JNEUROSCI.19-21-09587.1999
Synaptic basis of cortical persistent activity: the importance of NMDA receptors to working memory
Abstract
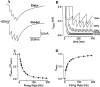
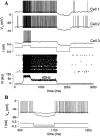
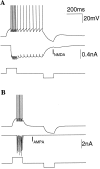
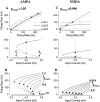
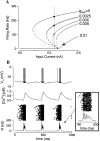
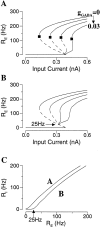
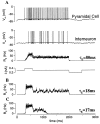
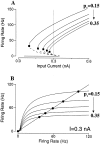
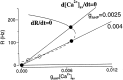
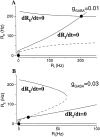
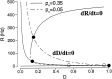
Delay-period activity of prefrontal cortical cells, the neural hallmark of working memory, is generally assumed to be sustained by reverberating synaptic excitation in the prefrontal cortical circuit. Previous model studies of working memory emphasized the high efficacy of recurrent synapses, but did not investigate the role of temporal synaptic dynamics. In this theoretical work, I show that biophysical properties of cortical synaptic transmission are important to the generation and stabilization of a network persistent state. This is especially the case when negative feedback mechanisms (such as spike-frequency adaptation, feedback shunting inhibition, and short-term depression of recurrent excitatory synapses) are included so that the neural firing rates are controlled within a physiological range (10-50 Hz), in spite of the exuberant recurrent excitation. Moreover, it is found that, to achieve a stable persistent state, recurrent excitatory synapses must be dominated by a slow component. If neuronal firings are asynchronous, the synaptic decay time constant needs to be comparable to that of the negative feedback; whereas in the case of partially synchronous dynamics, it needs to be comparable to a typical interspike interval (or oscillation period). Slow synaptic current kinetics also leads to the saturation of synaptic drive at high firing frequencies that contributes to rate control in a persistent state. For these reasons the slow NMDA receptor-mediated synaptic transmission is likely required for sustaining persistent network activity at low firing rates. This result suggests a critical role of the NMDA receptor channels in normal working memory function of the prefrontal cortex.
Figures



Similar articles
-
Effects of neuromodulation in a cortical network model of object working memory dominated by recurrent inhibition.J Comput Neurosci. 2001 Jul-Aug;11(1):63-85. doi: 10.1023/a:1011204814320. J Comput Neurosci. 2001. PMID: 11524578
-
The dynamical stability of reverberatory neural circuits.Biol Cybern. 2002 Dec;87(5-6):471-81. doi: 10.1007/s00422-002-0363-9. Biol Cybern. 2002. PMID: 12461636
-
Computer simulations of NMDA and non-NMDA receptor-mediated synaptic drive: sensory and supraspinal modulation of neurons and small networks.J Neurophysiol. 1993 Aug;70(2):695-709. doi: 10.1152/jn.1993.70.2.695. J Neurophysiol. 1993. PMID: 8105036
-
Implications of synaptic biophysics for recurrent network dynamics and active memory.Neural Netw. 2009 Oct;22(8):1189-200. doi: 10.1016/j.neunet.2009.07.016. Epub 2009 Jul 21. Neural Netw. 2009. PMID: 19647396 Review.
-
A synaptic basis for memory storage in the cerebral cortex.Proc Natl Acad Sci U S A. 1996 Nov 26;93(24):13453-9. doi: 10.1073/pnas.93.24.13453. Proc Natl Acad Sci U S A. 1996. PMID: 8942956 Free PMC article. Review.
Cited by
-
Ketamine disrupts naturalistic coding of working memory in primate lateral prefrontal cortex networks.Mol Psychiatry. 2021 Nov;26(11):6688-6703. doi: 10.1038/s41380-021-01082-5. Epub 2021 May 12. Mol Psychiatry. 2021. PMID: 33981008 Free PMC article.
-
Bistable, irregular firing and population oscillations in a modular attractor memory network.PLoS Comput Biol. 2010 Jun 3;6(6):e1000803. doi: 10.1371/journal.pcbi.1000803. PLoS Comput Biol. 2010. PMID: 20532199 Free PMC article.
-
Towards an executive without a homunculus: computational models of the prefrontal cortex/basal ganglia system.Philos Trans R Soc Lond B Biol Sci. 2007 Sep 29;362(1485):1601-13. doi: 10.1098/rstb.2007.2055. Philos Trans R Soc Lond B Biol Sci. 2007. PMID: 17428778 Free PMC article.
-
Late adolescent expression of GluN2B transmission in the prefrontal cortex is input-specific and requires postsynaptic protein kinase A and D1 dopamine receptor signaling.Biol Psychiatry. 2014 Mar 15;75(6):508-16. doi: 10.1016/j.biopsych.2013.07.033. Epub 2013 Sep 13. Biol Psychiatry. 2014. PMID: 24041503 Free PMC article.
-
Temporal Dynamics Underlying Perceptual Decision Making: Insights from the Interplay between an Attractor Model and Parietal Neurophysiology.Front Neurosci. 2008 Dec 15;2(2):245-54. doi: 10.3389/neuro.01.028.2008. eCollection 2008 Dec. Front Neurosci. 2008. PMID: 19225598 Free PMC article.
References
-
- Abbott LF. Realistic synaptic inputs for model neural networks. Network. 1991;2:245–258.
-
- Abbott LF, van Vreeswijk C. Asynchronous states in networks of pulse-coupled oscillators. Phys Rev. 1993;48:1483–1490. - PubMed
-
- Abbott LF, Varela JA, Sen K, Nelson SB. Synaptic depression and cortical gain control. Science. 1997;275:220–224. - PubMed
-
- Amari S. Dynamics of pattern formation in lateral-inhibition type neural fields. Biol Cybern. 1977;27:77–87. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
