Presenilin 1 controls gamma-secretase processing of amyloid precursor protein in pre-golgi compartments of hippocampal neurons
- PMID: 10525535
- PMCID: PMC2174229
- DOI: 10.1083/jcb.147.2.277
Presenilin 1 controls gamma-secretase processing of amyloid precursor protein in pre-golgi compartments of hippocampal neurons
Abstract
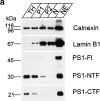
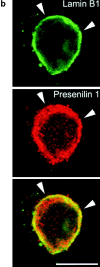
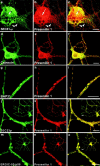
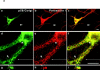
Mutations of presenilin 1 (PS1) causing Alzheimer's disease selectively increase the secretion of the amyloidogenic betaA4(1-42), whereas knocking out the gene results in decreased production of both betaA4(1-40) and (1-42) amyloid peptides (De Strooper et al. 1998). Therefore, PS1 function is closely linked to the gamma-secretase processing of the amyloid precursor protein (APP). Given the ongoing controversy on the subcellular localization of PS1, it remains unclear at what level of the secretory and endocytic pathways PS1 exerts its activity on APP and on the APP carboxy-terminal fragments that are the direct substrates for gamma-secretase. Therefore, we have reinvestigated the subcellular localization of endogenously expressed PS1 in neurons in vitro and in vivo using confocal microscopy and fine-tuned subcellular fractionation. We show that uncleaved PS1 holoprotein is recovered in the nuclear envelope fraction, whereas the cleaved PS fragments are found mainly in post-ER membranes including the intermediate compartment (IC). PS1 is concentrated in discrete sec23p- and p58/ERGIC-53-positive patches, suggesting its localization in subdomains involved in ER export. PS1 is not found to significant amounts beyond the cis-Golgi. Surprisingly, we found that APP carboxy-terminal fragments also coenrich in the pre-Golgi membrane fractions, consistent with the idea that these fragments are the real substrates for gamma-secretase. Functional evidence that PS1 exerts its effects on gamma-secretase processing of APP in the ER/IC was obtained using a series of APP trafficking mutants. These mutants were investigated in hippocampal neurons derived from transgenic mice expressing PS1wt or PS1 containing clinical mutations (PS1(M146L) and PS1(L286V)) at physiologically relevant levels. We demonstrate that the APP-London and PS1 mutations have additive effects on the increased secretion of betaA4(1-42) relative to betaA4(1-40), indicating that both mutations operate independently. Overall, our data clearly establish that PS1 controls gamma(42)-secretase activity in pre-Golgi compartments. We discuss models that reconcile this conclusion with the effects of PS1 deficiency on the generation of betaA4(1-40) peptide in the late biosynthetic and endocytic pathways.
Figures




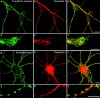

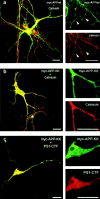
Similar articles
-
The discrepancy between presenilin subcellular localization and gamma-secretase processing of amyloid precursor protein.J Cell Biol. 2001 Aug 20;154(4):731-40. doi: 10.1083/jcb.200104045. Epub 2001 Aug 13. J Cell Biol. 2001. PMID: 11502763 Free PMC article.
-
Presenilin 1 regulates the processing of beta-amyloid precursor protein C-terminal fragments and the generation of amyloid beta-protein in endoplasmic reticulum and Golgi.Biochemistry. 1998 Nov 24;37(47):16465-71. doi: 10.1021/bi9816195. Biochemistry. 1998. PMID: 9843412
-
Presenilin complexes with the C-terminal fragments of amyloid precursor protein at the sites of amyloid beta-protein generation.Proc Natl Acad Sci U S A. 2000 Aug 1;97(16):9299-304. doi: 10.1073/pnas.97.16.9299. Proc Natl Acad Sci U S A. 2000. PMID: 10922078 Free PMC article.
-
Role of presenilin in gamma-secretase cleavage of amyloid precursor protein.Exp Gerontol. 2000 Jul;35(4):453-60. doi: 10.1016/s0531-5565(00)00111-x. Exp Gerontol. 2000. PMID: 10959033 Review.
-
Distinct presenilin-dependent and presenilin-independent gamma-secretases are responsible for total cellular Abeta production.J Neurosci Res. 2003 Nov 1;74(3):361-9. doi: 10.1002/jnr.10776. J Neurosci Res. 2003. PMID: 14598312 Review.
Cited by
-
Lysosomal calcium homeostasis defects, not proton pump defects, cause endo-lysosomal dysfunction in PSEN-deficient cells.J Cell Biol. 2012 Jul 9;198(1):23-35. doi: 10.1083/jcb.201201076. Epub 2012 Jul 2. J Cell Biol. 2012. PMID: 22753898 Free PMC article.
-
Upregulated function of mitochondria-associated ER membranes in Alzheimer disease.EMBO J. 2012 Nov 5;31(21):4106-23. doi: 10.1038/emboj.2012.202. Epub 2012 Aug 14. EMBO J. 2012. PMID: 22892566 Free PMC article.
-
The study of Golgi apparatus in Alzheimer's disease.Neurochem Res. 2007 Aug;32(8):1265-77. doi: 10.1007/s11064-007-9302-4. Epub 2007 Mar 31. Neurochem Res. 2007. PMID: 17401657 Review.
-
The calcium: an early signal that initiates the formation of the nervous system during embryogenesis.Front Mol Neurosci. 2012 May 14;5:3. doi: 10.3389/fnmol.2012.00064. eCollection 2012. Front Mol Neurosci. 2012. PMID: 22593733 Free PMC article.
-
The disintegrin/metalloproteinase ADAM10 is essential for the establishment of the brain cortex.J Neurosci. 2010 Apr 7;30(14):4833-44. doi: 10.1523/JNEUROSCI.5221-09.2010. J Neurosci. 2010. PMID: 20371803 Free PMC article.
References
-
- Arar C., Carpentier V., Le Caer J.P., Monsigny M., Legrand A., Roche A.C. ERGIC-53, a membrane protein of the endoplasmic reticulum-Golgi intermediate compartment, is identical to MR60, an intracellular mannose-specific lectin of myelomonocytic cells. J. Biol. Chem. 1995;270:3551–3553. - PubMed
-
- Bannykh S.I., Balch W.E. Selective transport of cargo between the endoplasmic reticulum and Golgi compartments. Histochem. Cell Biol. 1998;109:463–475. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

