Presenilin 2 deficiency causes a mild pulmonary phenotype and no changes in amyloid precursor protein processing but enhances the embryonic lethal phenotype of presenilin 1 deficiency
- PMID: 10518543
- PMCID: PMC18379
- DOI: 10.1073/pnas.96.21.11872
Presenilin 2 deficiency causes a mild pulmonary phenotype and no changes in amyloid precursor protein processing but enhances the embryonic lethal phenotype of presenilin 1 deficiency
Abstract
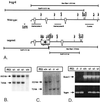
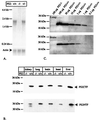
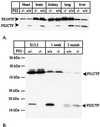
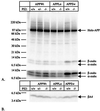
Mutations in the homologous presenilin 1 (PS1) and presenilin 2 (PS2) genes cause the most common and aggressive form of familial Alzheimer's disease. Although PS1 function and dysfunction have been extensively studied, little is known about the function of PS2 in vivo. To delineate the relationships of PS2 and PS1 activities and whether PS2 mutations involve gain or loss of function, we generated PS2 homozygous deficient (-/-) and PS1/PS2 double homozygous deficient mice. In contrast to PS1(-/-) mice, PS2(-/-) mice are viable and fertile and develop only mild pulmonary fibrosis and hemorrhage with age. Absence of PS2 does not detectably alter processing of amyloid precursor protein and has little or no effect on physiologically important apoptotic processes, indicating that Alzheimer's disease-causing mutations in PS2, as in PS1, result in gain of function. Although PS1(+/-) PS2( -/-) mice survive in relatively good health, complete deletion of both PS2 and PS1 genes causes a phenotype closely resembling full Notch-1 deficiency. These results demonstrate in vivo that PS1 and PS2 have partially overlapping functions and that PS1 is essential and PS2 is redundant for normal Notch signaling during mammalian embryological development.
Figures


Similar articles
-
Dissociated phenotypes in presenilin transgenic mice define functionally distinct gamma-secretases.Proc Natl Acad Sci U S A. 2005 Jun 21;102(25):8972-7. doi: 10.1073/pnas.0500940102. Epub 2005 Jun 10. Proc Natl Acad Sci U S A. 2005. PMID: 15951428 Free PMC article.
-
Regulation of amyloid precursor protein processing by presenilin 1 (PS1) and PS2 in PS1 knockout cells.J Biol Chem. 2000 Jan 7;275(1):215-22. doi: 10.1074/jbc.275.1.215. J Biol Chem. 2000. PMID: 10617607
-
A loss of function mutation of presenilin-2 interferes with amyloid beta-peptide production and notch signaling.J Biol Chem. 1999 Oct 1;274(40):28669-73. doi: 10.1074/jbc.274.40.28669. J Biol Chem. 1999. PMID: 10497236
-
Genes and mechanisms involved in beta-amyloid generation and Alzheimer's disease.Eur Arch Psychiatry Clin Neurosci. 1999;249(6):266-70. doi: 10.1007/s004060050098. Eur Arch Psychiatry Clin Neurosci. 1999. PMID: 10653281 Review.
-
Genes implicated in the pathogenesis of Alzheimer's disease.Front Biosci. 1997 Jun 1;2:d253-9. doi: 10.2741/a188. Front Biosci. 1997. PMID: 9206974 Review.
Cited by
-
Presenilin-2 and Calcium Handling: Molecules, Organelles, Cells and Brain Networks.Cells. 2020 Sep 25;9(10):2166. doi: 10.3390/cells9102166. Cells. 2020. PMID: 32992716 Free PMC article. Review.
-
RNAi-mediated inhibition of presenilin 2 inhibits glioma cell growth and invasion and is involved in the regulation of Nrg1/ErbB signaling.Neuro Oncol. 2012 Aug;14(8):994-1006. doi: 10.1093/neuonc/nos138. Epub 2012 Jun 29. Neuro Oncol. 2012. PMID: 22753229 Free PMC article.
-
Natural Modulators of Amyloid-Beta Precursor Protein Processing.Curr Alzheimer Res. 2012 Sep 13. Online ahead of print. Curr Alzheimer Res. 2012. PMID: 22998566 Free PMC article.
-
Alzheimer's disease-linked mutations in presenilin-1 result in a drastic loss of activity in purified γ-secretase complexes.PLoS One. 2012;7(4):e35133. doi: 10.1371/journal.pone.0035133. Epub 2012 Apr 18. PLoS One. 2012. PMID: 22529981 Free PMC article.
-
Selective suppression of oligodendrocyte-derived amyloid beta rescues neuronal dysfunction in Alzheimer's disease.PLoS Biol. 2024 Jul 23;22(7):e3002727. doi: 10.1371/journal.pbio.3002727. eCollection 2024 Jul. PLoS Biol. 2024. PMID: 39042667 Free PMC article.
References
-
- Doan A, Thinakaran G, Borchelt D R, Slunt H H, Ratovitsky T, Podlisny M, Selkoe D J, Seeger M, Gandy S E, Price D L, Sisodia S S. Neuron. 1996;17:1023–1030. - PubMed
-
- De Strooper B, Beullens M, Contreras B, Levesque L, Craessaerts K, Cordell B, Moechars D, Bollen M, Fraser P, George-Hyslop P S, Van Leuven F. J Biol Chem. 1997;272:3590–3598. - PubMed
-
- Rogaev E I, Sherrington R, Rogaeva E A, Levesque G, Ikeda M, Liang Y, Chi H, Lin C, Holman K, Tsuda T, et al. Nature (London) 1995;376:775–778. - PubMed
-
- Sherrington R, Rogaev E I, Liang Y, Rogaeva E A, Levesque G, Ikeda M, Chi H, Lin C, Li G, Holman K, et al. Nature (London) 1995;375:754–760. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

