Protofibrillar intermediates of amyloid beta-protein induce acute electrophysiological changes and progressive neurotoxicity in cortical neurons
- PMID: 10516307
- PMCID: PMC6782787
- DOI: 10.1523/JNEUROSCI.19-20-08876.1999
Protofibrillar intermediates of amyloid beta-protein induce acute electrophysiological changes and progressive neurotoxicity in cortical neurons
Abstract
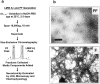
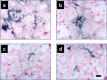
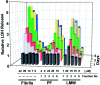
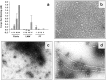
Alzheimer's disease (AD) is a progressive neurodegenerative disorder that is thought to be caused in part by the age-related accumulation of amyloid beta-protein (Abeta). The presence of neuritic plaques containing abundant Abeta-derived amyloid fibrils in AD brain tissue supports the concept that fibril accumulation per se underlies neuronal dysfunction in AD. Recent observations have begun to challenge this assumption by suggesting that earlier Abeta assemblies formed during the process of fibrillogenesis may also play a role in AD pathogenesis. Here, we present the novel finding that protofibrils (PF), metastable intermediates in amyloid fibril formation, can alter the electrical activity of neurons and cause neuronal loss. Both low molecular weight Abeta (LMW Abeta) and PF reproducibly induced toxicity in mixed brain cultures in a time- and concentration-dependent manner. No increase in fibril formation during the course of the experiments was observed by either Congo red binding or electron microscopy, suggesting that the neurotoxicity of LMW Abeta and PF cannot be explained by conversion to fibrils. Importantly, protofibrils, but not LMW Abeta, produced a rapid increase in EPSPs, action potentials, and membrane depolarizations. These data suggest that PF have inherent biological activity similar to that of mature fibrils. Our results raise the possibility that the preclinical and early clinical progression of AD is driven in part by the accumulation of specific Abeta assembly intermediates formed during the process of fibrillogenesis.
Figures


Similar articles
-
Α-mangostin, a polyphenolic xanthone derivative from mangosteen, attenuates β-amyloid oligomers-induced neurotoxicity by inhibiting amyloid aggregation.Neuropharmacology. 2012 Feb;62(2):871-81. doi: 10.1016/j.neuropharm.2011.09.016. Epub 2011 Sep 24. Neuropharmacology. 2012. PMID: 21958557
-
Neuronal fibrillogenesis: amyloid fibrils from primary neuronal cultures impair long-term memory in the crab Chasmagnathus.Behav Brain Res. 2003 Dec 17;147(1-2):73-82. doi: 10.1016/s0166-4328(03)00118-9. Behav Brain Res. 2003. PMID: 14659572
-
Amyloid beta-protein dimers rapidly form stable synaptotoxic protofibrils.J Neurosci. 2010 Oct 27;30(43):14411-9. doi: 10.1523/JNEUROSCI.3537-10.2010. J Neurosci. 2010. PMID: 20980598 Free PMC article.
-
Alzheimer's disease.Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
-
Amyloid fibril toxicity in Alzheimer's disease and diabetes.Ann N Y Acad Sci. 1996 Jan 17;777:89-95. doi: 10.1111/j.1749-6632.1996.tb34406.x. Ann N Y Acad Sci. 1996. PMID: 8624132 Review.
Cited by
-
Innovative Therapeutic Strategies in Alzheimer's Disease: A Synergistic Approach to Neurodegenerative Disorders.Pharmaceuticals (Basel). 2024 Jun 6;17(6):741. doi: 10.3390/ph17060741. Pharmaceuticals (Basel). 2024. PMID: 38931409 Free PMC article. Review.
-
Tailoring the antibody response to aggregated Aß using novel Alzheimer-vaccines.PLoS One. 2015 Jan 22;10(1):e0115237. doi: 10.1371/journal.pone.0115237. eCollection 2015. PLoS One. 2015. PMID: 25611858 Free PMC article.
-
PGH2-derived levuglandin adducts increase the neurotoxicity of amyloid beta1-42.J Neurochem. 2006 Feb;96(4):917-23. doi: 10.1111/j.1471-4159.2005.03586.x. Epub 2006 Jan 12. J Neurochem. 2006. PMID: 16412101 Free PMC article.
-
Nanotools for megaproblems: probing protein misfolding diseases using nanomedicine modus operandi.J Proteome Res. 2006 Oct;5(10):2505-22. doi: 10.1021/pr0603349. J Proteome Res. 2006. PMID: 17022621 Free PMC article. Review.
-
Size-dependent neurotoxicity of beta-amyloid oligomers.Arch Biochem Biophys. 2010 Apr 15;496(2):84-92. doi: 10.1016/j.abb.2010.02.001. Epub 2010 Feb 11. Arch Biochem Biophys. 2010. PMID: 20153288 Free PMC article.
References
-
- Aksenova MV, Aksenov MY, Butterfield DA, Carney JM. α-1-antichymotrypsin interaction with Aβ (1–40) inhibits fibril formation but does not affect the peptide toxicity. Neurosci Lett. 1996;211:45–48. - PubMed
-
- Anderton BH, Callahan L, Coleman P, Davies P, Flood D, Jicha GA, Ohm T, Weaver C. Dendritic changes in Alzheimer’s disease and factors that may underlie these changes. Prog Neurobiol. 1998;55:595–609. - PubMed
-
- Chapman PF, White GL, Jones MW, Cooper-Blacketer D, Marshall VJ, Irizarry M, Younkin L, Good MA, Bliss TV, Hyman BT, Younkin SG, Hsiao KK. Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nat Neurosci. 1999;2:271–276. - PubMed
-
- Cummings JL, Vinters HV, Cole GM, Khachaturian ZS. Alzheimer’s disease: etiologies, pathophysiology, cognitive reserve, and treatment opportunities. Neurology. 1998;51:S2–S17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
