The Saccharomyces cerevisiae homologue of human Wiskott-Aldrich syndrome protein Las17p interacts with the Arp2/3 complex
- PMID: 10512884
- PMCID: PMC25621
- DOI: 10.1091/mbc.10.10.3521
The Saccharomyces cerevisiae homologue of human Wiskott-Aldrich syndrome protein Las17p interacts with the Arp2/3 complex
Abstract
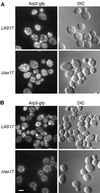
Yeast Las17 protein is homologous to the Wiskott-Aldrich Syndrome protein, which is implicated in severe immunodeficiency. Las17p/Bee1p has been shown to be important for actin patch assembly and actin polymerization. Here we show that Las17p interacts with the Arp2/3 complex. LAS17 is an allele-specific multicopy suppressor of ARP2 and ARP3 mutations; overexpression restores both actin patch organization and endocytosis defects in ARP2 temperature-sensitive (ts) cells. Six of seven ARP2 ts mutants and at least one ARP3 ts mutant are synthetically lethal with las17Delta ts confirming functional interaction with the Arp2/3 complex. Further characterization of las17Delta cells showed that receptor-mediated internalization of alpha factor by the Ste2 receptor is severely defective. The polarity of normal bipolar bud site selection is lost. Las17-gfp remains localized in cortical patches in vivo independently of polymerized actin and is required for the polarized localization of Arp2/3 as well as actin. Coimmunoprecipitation of Arp2p with Las17p indicates that Las17p interacts directly with the complex. Two hybrid results also suggest that Las17p interacts with actin, verprolin, Rvs167p and several other proteins including Src homology 3 (SH3) domain proteins, suggesting that Las17p may integrate signals from different regulatory cascades destined for the Arp2/3p complex and the actin cytoskeleton.
Figures



Similar articles
-
Saccharomyces cerevisiae Bzz1p is implicated with type I myosins in actin patch polarization and is able to recruit actin-polymerizing machinery in vitro.Mol Cell Biol. 2002 Nov;22(22):7889-906. doi: 10.1128/MCB.22.22.7889-7906.2002. Mol Cell Biol. 2002. PMID: 12391157 Free PMC article.
-
The WASp homologue Las17p functions with the WIP homologue End5p/verprolin and is essential for endocytosis in yeast.Curr Biol. 1998 Aug 27;8(17):959-62. doi: 10.1016/s0960-9822(98)70396-3. Curr Biol. 1998. PMID: 9742397
-
Activation of the yeast Arp2/3 complex by Bee1p, a WASP-family protein.Curr Biol. 1999 May 6;9(9):501-4. doi: 10.1016/s0960-9822(99)80218-8. Curr Biol. 1999. PMID: 10322115
-
WASP family proteins, more than Arp2/3 activators.Biochem Soc Trans. 2016 Oct 15;44(5):1339-1345. doi: 10.1042/BST20160176. Biochem Soc Trans. 2016. PMID: 27911716 Free PMC article. Review.
-
Regulation of actin polymerization by Arp2/3 complex and WASp/Scar proteins.J Biol Chem. 1999 Nov 12;274(46):32531-4. doi: 10.1074/jbc.274.46.32531. J Biol Chem. 1999. PMID: 10551802 Review. No abstract available.
Cited by
-
Targeting and functional mechanisms of the cytokinesis-related F-BAR protein Hof1 during the cell cycle.Mol Biol Cell. 2013 May;24(9):1305-20. doi: 10.1091/mbc.E12-11-0804. Epub 2013 Mar 6. Mol Biol Cell. 2013. PMID: 23468521 Free PMC article.
-
Evolution of the SH3 Domain Specificity Landscape in Yeasts.PLoS One. 2015 Jun 11;10(6):e0129229. doi: 10.1371/journal.pone.0129229. eCollection 2015. PLoS One. 2015. PMID: 26068101 Free PMC article.
-
Functions of Vrp1p in cytokinesis and actin patches are distinct and neither requires a WH2/V domain.EMBO J. 2001 Dec 17;20(24):6979-89. doi: 10.1093/emboj/20.24.6979. EMBO J. 2001. PMID: 11742975 Free PMC article.
-
Lsb5p interacts with actin regulators Sla1p and Las17p, ubiquitin and Arf3p to couple actin dynamics to membrane trafficking processes.Biochem J. 2005 May 1;387(Pt 3):649-58. doi: 10.1042/BJ20041729. Biochem J. 2005. PMID: 15651983 Free PMC article.
-
Physiological and environmental control of yeast prions.FEMS Microbiol Rev. 2014 Mar;38(2):326-44. doi: 10.1111/1574-6976.12053. Epub 2013 Dec 4. FEMS Microbiol Rev. 2014. PMID: 24236638 Free PMC article. Review.
References
-
- Amberg DC, Basart E, Botstein D. Defining protein interaction with yeast actin in vivo. Nat Struct Biol. 1995;2:28–35. - PubMed
-
- Amman AJ, Hong R. Disorders of the T-cell system. In: Stiehm ER, editor. Immunological Disorders in Children. Philadelphia: W.B. Saunders; 1989. pp. 257–315.
-
- Apenström P, Lindberg U, Hall A. Two GTPases, Cdc42 and Rac, bind directly to a protein implicated in the immunodeficiency disorder Wiskott–Aldrich syndrome. Curr Biol. 1996;6:70–75. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

