Human major group rhinoviruses downmodulate the accessory function of monocytes by inducing IL-10
- PMID: 10510336
- PMCID: PMC408557
- DOI: 10.1172/JCI7255
Human major group rhinoviruses downmodulate the accessory function of monocytes by inducing IL-10
Abstract
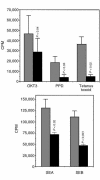
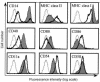
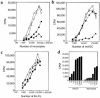
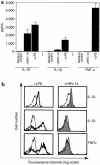
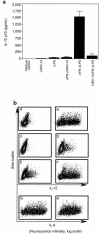
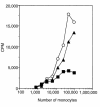
Human rhinoviruses (HRVs) are the predominant cause of the common cold. Although this disease is per se rather harmless, HRV infection is considered to set the stage for more dangerous pathogens in vivo. Here we demonstrate that HRV-14, a member of the major group HRV family, can efficiently inhibit antigen-induced T-cell proliferation and T-cell responses to allogeneic monocytes. HRV-14 triggered a significant downregulation of MHC class II molecules on monocytes. Moreover, supernatants from monocytes cultured in the presence of HRV-14 strongly reduced the allogeneic T-cell stimulatory property of untreated monocytes and monocyte-derived dendritic cells (md-DCs), whereas Epstein Barr virus-transformed B-lymphoblastoid cells were not sensitive. Analysis of the supernatant revealed that HRV-14 induced the production of significant amounts of the immunosuppressive cytokine IL-10. The important T-cell stimulatory cytokine IL-12 or the proinflammatory cytokines IL-1beta or TNF-alpha were not detected or were only minimally detected. Finally, monocytes pretreated with HRV-14 were greatly inhibited in their production of IL-12 upon stimulation with IFN-gamma/LPS. These observations suggest that altered cytokine production in mononuclear phagocytes upon interaction with HRV downmodulates appropriate immune responses during the viral infection.
Figures

Similar articles
-
IL-4 decreases the expression of the monocyte differentiation marker CD14, paralleled by an increasing accessory potency.Immunobiology. 1991 Aug;182(5):449-64. doi: 10.1016/S0171-2985(11)80209-3. Immunobiology. 1991. PMID: 1717365
-
Tumor necrosis factor alpha and CD40 ligand antagonize the inhibitory effects of interleukin 10 on T-cell stimulatory capacity of dendritic cells.Cancer Res. 2000 Aug 15;60(16):4485-92. Cancer Res. 2000. PMID: 10969796
-
Cytokine stimulation of T lymphocytes regulates their capacity to induce monocyte production of tumor necrosis factor-alpha, but not interleukin-10: possible relevance to pathophysiology of rheumatoid arthritis.Eur J Immunol. 1997 Mar;27(3):624-32. doi: 10.1002/eji.1830270308. Eur J Immunol. 1997. PMID: 9079801
-
Interleukin-10.Annu Rev Immunol. 1993;11:165-90. doi: 10.1146/annurev.iy.11.040193.001121. Annu Rev Immunol. 1993. PMID: 8386517 Review.
-
Functional characterization of human IL-10.Int Arch Allergy Immunol. 1992;99(1):8-15. doi: 10.1159/000236329. Int Arch Allergy Immunol. 1992. PMID: 1336421 Review.
Cited by
-
Respiratory viral infections in infants: causes, clinical symptoms, virology, and immunology.Clin Microbiol Rev. 2010 Jan;23(1):74-98. doi: 10.1128/CMR.00032-09. Clin Microbiol Rev. 2010. PMID: 20065326 Free PMC article. Review.
-
Solution structure of the X4 protein coded by the SARS related coronavirus reveals an immunoglobulin like fold and suggests a binding activity to integrin I domains.J Biomed Sci. 2006 May;13(3):281-93. doi: 10.1007/s11373-005-9043-9. Epub 2005 Nov 23. J Biomed Sci. 2006. PMID: 16328780 Free PMC article.
-
A longitudinal study of gene expression in healthy individuals.BMC Med Genomics. 2009 Jun 7;2:33. doi: 10.1186/1755-8794-2-33. BMC Med Genomics. 2009. PMID: 19500411 Free PMC article.
-
Human monocytic cells direct the robust release of CXCL10 by bronchial epithelial cells during rhinovirus infection.Clin Exp Allergy. 2010 Aug;40(8):1203-13. doi: 10.1111/j.1365-2222.2010.03546.x. Epub 2010 Jun 7. Clin Exp Allergy. 2010. PMID: 20545701 Free PMC article.
-
Infection of murine keratinocytes with herpes simplex virus type 1 induces the expression of interleukin-10, but not interleukin-1 alpha or tumour necrosis factor-alpha.Immunology. 2001 Dec;104(4):468-75. doi: 10.1046/j.1365-2567.2001.01330.x. Immunology. 2001. PMID: 11899434 Free PMC article.
References
-
- Couch, R. 1996. Rhinoviruses. In Fields virology. B. Fields et al., editors. Lippincott-Raven Publishers. Philadelphia, PA. 713–734.
-
- Pitkäranta A, Hayden FG. What’s new with common colds? Pathogenesis and diagnosis. Infect Med. 1998;15:50–59.
-
- Turner RB, Hendley JO, Gwaltney JM., Jr Shedding of infected ciliated epithelial cells in rhinovirus colds. J Infect Dis. 1982;145:849–853. - PubMed
-
- Winther B, Brofeldt S, Christensen B, Mygind N. Light and scanning electron microscopy of nasal biopsy material from patients with naturally acquired common colds. Acta Otolaryngol (Stockh) 1984;97:309–318. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

