Quinolone signaling in the cell-to-cell communication system of Pseudomonas aeruginosa
- PMID: 10500159
- PMCID: PMC18016
- DOI: 10.1073/pnas.96.20.11229
Quinolone signaling in the cell-to-cell communication system of Pseudomonas aeruginosa
Abstract
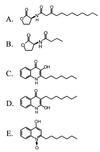
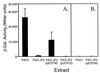
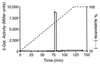
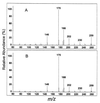
Numerous species of bacteria use an elegant regulatory mechanism known as quorum sensing to control the expression of specific genes in a cell-density dependent manner. In Gram-negative bacteria, quorum sensing systems function through a cell-to-cell signal molecule (autoinducer) that consists of a homoserine lactone with a fatty acid side chain. Such is the case in the opportunistic human pathogen Pseudomonas aeruginosa, which contains two quorum sensing systems (las and rhl) that operate via the autoinducers, N-(3-oxododecanoyl)-L-homoserine lactone and N-butyryl-L-homoserine lactone. The study of these signal molecules has shown that they bind to and activate transcriptional activator proteins that specifically induce numerous P. aeruginosa virulence genes. We report here that P. aeruginosa produces another signal molecule, 2-heptyl-3-hydroxy-4-quinolone, which has been designated as the Pseudomonas quinolone signal. It was found that this unique cell-to-cell signal controlled the expression of lasB, which encodes for the major virulence factor, LasB elastase. We also show that the synthesis and bioactivity of Pseudomonas quinolone signal were mediated by the P. aeruginosa las and rhl quorum sensing systems, respectively. The demonstration that 2-heptyl-3-hydroxy-4-quinolone can function as an intercellular signal sheds light on the role of secondary metabolites and shows that P. aeruginosa cell-to-cell signaling is not restricted to acyl-homoserine lactones.
Figures
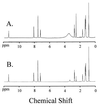
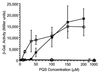
Comment in
-
Novel antimicrobial targets from combined pathogen and host genetics.Proc Natl Acad Sci U S A. 2000 Feb 1;97(3):958-9. doi: 10.1073/pnas.97.3.958. Proc Natl Acad Sci U S A. 2000. PMID: 10655466 Free PMC article. No abstract available.
Similar articles
-
The Pseudomonas quinolone signal regulates rhl quorum sensing in Pseudomonas aeruginosa.J Bacteriol. 2000 May;182(10):2702-8. doi: 10.1128/JB.182.10.2702-2708.2000. J Bacteriol. 2000. PMID: 10781536 Free PMC article.
-
Interference with Pseudomonas quinolone signal synthesis inhibits virulence factor expression by Pseudomonas aeruginosa.Proc Natl Acad Sci U S A. 2001 Sep 25;98(20):11633-7. doi: 10.1073/pnas.201328498. Proc Natl Acad Sci U S A. 2001. PMID: 11573001 Free PMC article.
-
Dueling quorum sensing systems in Pseudomonas aeruginosa control the production of the Pseudomonas quinolone signal (PQS).FEMS Microbiol Lett. 2004 Jan 15;230(1):27-34. doi: 10.1016/S0378-1097(03)00849-8. FEMS Microbiol Lett. 2004. PMID: 14734162
-
The third quorum-sensing system of Pseudomonas aeruginosa: Pseudomonas quinolone signal and the enigmatic PqsE protein.J Med Microbiol. 2020 Jan;69(1):25-34. doi: 10.1099/jmm.0.001116. J Med Microbiol. 2020. PMID: 31794380 Review.
-
Quorum sensing by 2-alkyl-4-quinolones in Pseudomonas aeruginosa and other bacterial species.Mol Biosyst. 2008 Sep;4(9):882-8. doi: 10.1039/b803796p. Epub 2008 Jun 30. Mol Biosyst. 2008. PMID: 18704225 Review.
Cited by
-
The extracellular death factor (EDF) protects Escherichia coli by scavenging hydroxyl radicals induced by bactericidal antibiotics.Springerplus. 2015 Apr 16;4:182. doi: 10.1186/s40064-015-0968-9. eCollection 2015. Springerplus. 2015. PMID: 25932369 Free PMC article.
-
A cell-cell communication signal integrates quorum sensing and stress response.Nat Chem Biol. 2013 May;9(5):339-43. doi: 10.1038/nchembio.1225. Epub 2013 Mar 31. Nat Chem Biol. 2013. PMID: 23542643
-
Moonlighting chaperone activity of the enzyme PqsE contributes to RhlR-controlled virulence of Pseudomonas aeruginosa.Nat Commun. 2022 Dec 1;13(1):7402. doi: 10.1038/s41467-022-35030-w. Nat Commun. 2022. PMID: 36456567 Free PMC article.
-
The Pseudomonas aeruginosa extracellular secondary metabolite, Paerucumarin, chelates iron and is not localized to extracellular membrane vesicles.J Microbiol. 2016 Aug;54(8):573-81. doi: 10.1007/s12275-016-5645-3. Epub 2016 Aug 2. J Microbiol. 2016. PMID: 27480638
-
The transcriptional regulator CzcR modulates antibiotic resistance and quorum sensing in Pseudomonas aeruginosa.PLoS One. 2012;7(5):e38148. doi: 10.1371/journal.pone.0038148. Epub 2012 May 29. PLoS One. 2012. PMID: 22666466 Free PMC article.
References
-
- Fuqua W C, Winans S C, Greenberg E P. Annu Rev Microbiol. 1996;50:727–751. - PubMed
-
- Pesci E C, Iglewski B H. In: Cell-Cell Signaling in Bacteria. Dunny G, Winans S C, editors. Washington, DC: Am. Soc. Microbiol.; 1999. pp. 147–155.
-
- Passador L, Cook J M, Gambello M J, Rust L, Iglewski B H. Science. 1993;260:1127–1130. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

