Decreased G-protein-mediated regulation and shift in calcium channel types with age in hippocampal cultures
- PMID: 10493768
- PMCID: PMC6783042
- DOI: 10.1523/JNEUROSCI.19-19-08674.1999
Decreased G-protein-mediated regulation and shift in calcium channel types with age in hippocampal cultures
Abstract
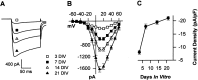
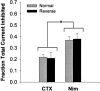
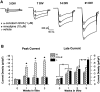
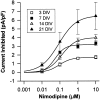
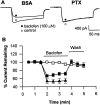
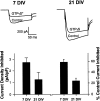
The membrane density of L-type voltage-sensitive Ca(2+) channels (L-VSCCs) of rat hippocampal neurons increases over age [days in vitro (DIV)] in long-term primary cultures, apparently contributing both to spontaneous cell death and to enhanced excitotoxic vulnerability. Similar increases in L-VSCCs occur during brain aging in vivo in rat and rabbit hippocampal neurons. However, unraveling both the molecular basis and the functional implications of these age changes in VSCC density will require determining whether the other types of high-threshold VSCCs (e.g., N, P/Q, and R) also exhibit altered density and/or changes in regulation, for example, by the important G-protein-coupled, membrane-delimited inhibitory pathway. These possibilities were tested here in long-term hippocampal cultures. Pharmacologically defined whole-cell currents were corrected for cell size differences over age by normalization with whole-cell capacitance. The Ca(2+) channel current density (picoamperes per picofarad), mediated by each Ca(2+) channel type studied here (L, N, and a combined P/Q + R component), increased through 7 DIV. Thereafter, however, only L-type current density continued to increase, at least through 21 DIV. Concurrently, pertussis toxin-sensitive G-protein-coupled inhibition of non-L-type Ca(2+) channel current induced by the GABA(B) receptor agonist baclofen or by guanosine 5'-3-O-(thio)triphosphate declined dramatically with age in culture. Thus, the present studies identify selective and novel parallel mechanisms for the time-dependent alteration of Ca(2+) influx, which could importantly influence function and vulnerability during development and/or aging.
Figures

Similar articles
-
Modulation of calcium currents by a metabotropic glutamate receptor involves fast and slow kinetic components in cultured hippocampal neurons.J Neurosci. 1993 Jul;13(7):3041-50. doi: 10.1523/JNEUROSCI.13-07-03041.1993. J Neurosci. 1993. PMID: 8392538 Free PMC article.
-
High-voltage-activated calcium currents in neurons acutely isolated from the ventrobasal nucleus of the rat thalamus.J Neurophysiol. 1997 Jan;77(1):465-75. doi: 10.1152/jn.1997.77.1.465. J Neurophysiol. 1997. PMID: 9120587
-
TXA2 agonists inhibit high-voltage-activated calcium channels in rat hippocampal CA1 neurons.Am J Physiol. 1996 Oct;271(4 Pt 1):C1269-77. doi: 10.1152/ajpcell.1996.271.4.C1269. Am J Physiol. 1996. PMID: 8897834
-
Mu-opioid and GABA(B) receptors modulate different types of Ca2+ currents in rat nodose ganglion neurons.Neuroscience. 1998 Aug;85(3):939-56. doi: 10.1016/s0306-4522(97)00674-x. Neuroscience. 1998. PMID: 9639286
-
Gamma-aminobutyric acid type B receptors facilitate L-type and attenuate N-type Ca(2+) currents in isolated hippocampal neurons.J Neurosci Res. 2004 May 1;76(3):323-33. doi: 10.1002/jnr.20085. J Neurosci Res. 2004. PMID: 15079861
Cited by
-
Modulation of L-type calcium channels in Alzheimer's disease: A potential therapeutic target.Comput Struct Biotechnol J. 2022 Nov 26;21:11-20. doi: 10.1016/j.csbj.2022.11.049. eCollection 2023. Comput Struct Biotechnol J. 2022. PMID: 36514335 Free PMC article. Review.
-
Novel calcium-related targets of insulin in hippocampal neurons.Neuroscience. 2017 Nov 19;364:130-142. doi: 10.1016/j.neuroscience.2017.09.019. Epub 2017 Sep 20. Neuroscience. 2017. PMID: 28939258 Free PMC article.
-
Analysis of gene expression during aging of CGNs in culture: implication of SLIT2 and NPY in senescence.Age (Dordr). 2015 Jun;37(3):62. doi: 10.1007/s11357-015-9789-6. Epub 2015 Jun 6. Age (Dordr). 2015. PMID: 26047956 Free PMC article.
-
Functional consequences of age-related morphologic changes to pyramidal neurons of the rhesus monkey prefrontal cortex.J Comput Neurosci. 2015 Apr;38(2):263-83. doi: 10.1007/s10827-014-0541-5. Epub 2014 Dec 20. J Comput Neurosci. 2015. PMID: 25527184 Free PMC article.
-
Functional implications of a psychiatric risk variant within CACNA1C in induced human neurons.Mol Psychiatry. 2015 Feb;20(2):162-9. doi: 10.1038/mp.2014.143. Epub 2014 Nov 18. Mol Psychiatry. 2015. PMID: 25403839 Free PMC article.
References
-
- Abele AE, Scholz KP, Scholz WK, Miller RJ. Excitotoxicity induced by enhanced excitatory neurotransmission in cultured hippocampal pyramidal neurons. Neuron. 1990;2:413–419. - PubMed
-
- Adamec C, Didier M, Nixon RA. Developmental regulation of the recovery process following glutamate-induced calcium rise in rodent primary neuronal cultures. Brain Res Dev Brain Res. 1998;108:101–110. - PubMed
-
- Ankarcrona M, Dypbukt JM, Bonfoco E, Zhitovsky E, Orrenius S, Lipton SA, Nicotera P. Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron. 1995;15:961–973. - PubMed
-
- Armitage P, Berry G. Statistical methods in scientific research. Blackwell Scientific; Boston: 1990.
-
- Armstrong CM, Gilly WF. Access resistance and space-clamp problems associated with whole-cell patch clamping. Methods Enzymol. 1992;207:100–122. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
