Inhibition of human immunodeficiency virus type 1 infectivity by the gp41 core: role of a conserved hydrophobic cavity in membrane fusion
- PMID: 10482611
- PMCID: PMC112878
- DOI: 10.1128/JVI.73.10.8578-8586.1999
Inhibition of human immunodeficiency virus type 1 infectivity by the gp41 core: role of a conserved hydrophobic cavity in membrane fusion
Abstract
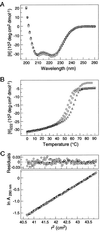
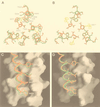
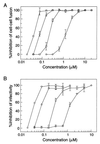
The gp41 envelope protein of human immunodeficiency virus type 1 (HIV-1) contains an alpha-helical core structure responsible for mediating membrane fusion during viral entry. Recent studies suggest that a conserved hydrophobic cavity in the coiled coil of this core plays a distinctive structural role in maintaining the fusogenic conformation of the gp41 molecule. Here we investigated the importance of this cavity in determining the structure and biological activity of the gp41 core by using the N34(L6)C28 model. The high-resolution crystal structures of N34(L6)C28 of two HIV-1 gp41 fusion-defective mutants reveal that each mutant sequence is accommodated in the six-helix bundle structure by forming the cavity with different sets of atoms. Remarkably, the mutant N34(L6)C28 cores are highly effective inhibitors of HIV-1 infection, with 5- to 16-fold greater activity than the wild-type molecule. The enhanced inhibitory activity by fusion-defective mutations correlates with local structural perturbations close to the cavity that destabilize the six-helix bundle. Taken together, these results indicate that the conserved hydrophobic coiled-coil cavity in the gp41 core is critical for HIV-1 entry and its inhibition and provides a potential antiviral drug target.
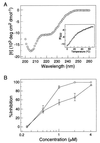
Figures


Similar articles
-
Helical interactions in the HIV-1 gp41 core reveal structural basis for the inhibitory activity of gp41 peptides.Biochemistry. 2000 Feb 22;39(7):1634-42. doi: 10.1021/bi9921687. Biochemistry. 2000. PMID: 10677212
-
Subdomain folding and biological activity of the core structure from human immunodeficiency virus type 1 gp41: implications for viral membrane fusion.J Virol. 1999 May;73(5):4433-8. doi: 10.1128/JVI.73.5.4433-4438.1999. J Virol. 1999. PMID: 10196341 Free PMC article.
-
Interactions between HIV-1 gp41 core and detergents and their implications for membrane fusion.J Biol Chem. 2000 Jan 21;275(3):1839-45. doi: 10.1074/jbc.275.3.1839. J Biol Chem. 2000. PMID: 10636883
-
N- and C-domains of HIV-1 gp41: mutation, structure and functions.Immunol Lett. 2001 Jan 15;75(3):215-20. doi: 10.1016/s0165-2478(00)00302-3. Immunol Lett. 2001. PMID: 11166378 Review.
-
Development of HIV entry inhibitors targeted to the coiled-coil regions of gp41.Biochem Biophys Res Commun. 2000 Mar 24;269(3):641-6. doi: 10.1006/bbrc.1999.1972. Biochem Biophys Res Commun. 2000. PMID: 10720469 Review.
Cited by
-
Insights into vaccine development for acquired immune deficiency syndrome from crystal structures of human immunodeficiency virus-1 gp41 and equine infectious anemia virus gp45.Protein Sci. 2015 Oct;24(10):1549-59. doi: 10.1002/pro.2750. Epub 2015 Sep 21. Protein Sci. 2015. PMID: 26174372 Free PMC article. Review.
-
Conserved salt bridge between the N- and C-terminal heptad repeat regions of the human immunodeficiency virus type 1 gp41 core structure is critical for virus entry and inhibition.J Virol. 2008 Nov;82(22):11129-39. doi: 10.1128/JVI.01060-08. Epub 2008 Sep 3. J Virol. 2008. PMID: 18768964 Free PMC article.
-
Discovery of Highly Potent Small Molecule Pan-Coronavirus Fusion Inhibitors.Viruses. 2023 Apr 19;15(4):1001. doi: 10.3390/v15041001. Viruses. 2023. PMID: 37112982 Free PMC article.
-
Comparison of predicted scaffold-compatible sequence variation in the triple-hairpin structure of human imunodeficiency virus type 1 gp41 with patient data.J Virol. 2002 Aug;76(15):7595-606. doi: 10.1128/jvi.76.15.7595-7606.2002. J Virol. 2002. PMID: 12097573 Free PMC article.
-
Rationally designed anti-HIV peptides containing multifunctional domains as molecule probes for studying the mechanisms of action of the first and second generation HIV fusion inhibitors.J Biol Chem. 2008 Oct 31;283(44):30376-84. doi: 10.1074/jbc.M804672200. Epub 2008 Jul 28. J Biol Chem. 2008. PMID: 18662985 Free PMC article.
References
-
- Brünger A T. XPLOR version 3.1: a system for X-ray crystallography and NMR. New Harven, Conn: Yale University Press; 1992.
-
- Bullough P A, Hughson F M, Skehel J J, Wiley D C. Structure of influenza hemagglutinin at the pH of membrane fusion. Nature. 1994;371:37–43. - PubMed
-
- Cantor C, Schimmel P. Biophysical chemistry, part III. New York, N.Y: W. H. Freeman and Company; 1980. pp. 1131–1132.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

