Mice lacking all conventional MHC class II genes
- PMID: 10468609
- PMCID: PMC17889
- DOI: 10.1073/pnas.96.18.10338
Mice lacking all conventional MHC class II genes
Abstract
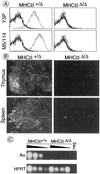
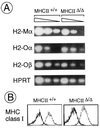
MHC class II (MHC-II) molecules play a central role in the selection of the T cell repertoire, in the establishment and regulation of the adaptive immune response, and in autoimmune deviation. We have generated knockout mice lacking all four of the classical murine MHC-II genes (MHCII(Delta/Delta) mice), via a large (80-kilobase) deletion of the entire class II region that was engineered by homologous recombination and Cre recombinase-mediated excision. These mice feature immune system perturbations like those of Aalpha and Abeta knockout animals, notably a dearth of CD4(+) lymphocytes in the thymus and spleen. No new anatomical or physiological abnormalities were observed in MHCII(Delta/Delta) mice. Because these animals are devoid of all classical MHC-II chains, even unpaired chains, they make excellent recipients for MHC-II transgenes from other species, avoiding the problem of interspecies cross-pairing of MHC-II chains. Therefore, they should be invaluable for engineering "humanized" mouse models of human MHC-II-associated autoimmune disorders.
Figures



Similar articles
-
Characterization of HLA DR2 and DQ8 transgenic mouse with a new engineered mouse class II deletion, which lacks all endogenous class II genes.J Autoimmun. 2003 Nov;21(3):195-9. doi: 10.1016/s0896-8411(03)00120-3. J Autoimmun. 2003. PMID: 14599844
-
[Autoimmune-associated MHC class II molecules].Hautarzt. 1995 Apr;46(4):225-7. doi: 10.1007/s001050050244. Hautarzt. 1995. PMID: 7540603 Review. German.
-
Developmental dissociation of T cells from B, NK, and myeloid cells revealed by MHC class II-specific chimeric immune receptors bearing TCR-zeta or FcR-gamma chain signaling domains.Blood. 2002 Oct 15;100(8):3045-8. doi: 10.1182/blood-2002-02-0428. Blood. 2002. PMID: 12351421
-
Transient T and B cell activation after neonatal induction of tolerance to MHC class II or Mls alloantigens.J Immunol. 1991 Apr 1;146(7):2152-60. J Immunol. 1991. PMID: 1672344
-
Function and regulation of MHC class II molecules in T-lymphocytes: of mice and men.Hum Immunol. 2004 Apr;65(4):282-90. doi: 10.1016/j.humimm.2004.01.005. Hum Immunol. 2004. PMID: 15120183 Review.
Cited by
-
Host glycans and antigen presentation.Microbes Infect. 2012 Sep;14(11):894-903. doi: 10.1016/j.micinf.2012.04.010. Epub 2012 Apr 22. Microbes Infect. 2012. PMID: 22580092 Free PMC article. Review.
-
Class II major histocompatibility complex plays an essential role in obesity-induced adipose inflammation.Cell Metab. 2013 Mar 5;17(3):411-22. doi: 10.1016/j.cmet.2013.02.009. Cell Metab. 2013. PMID: 23473035 Free PMC article.
-
Low expression of RNA sensors impacts Zika virus infection in the lower female reproductive tract.Nat Commun. 2019 Sep 25;10(1):4344. doi: 10.1038/s41467-019-12371-7. Nat Commun. 2019. PMID: 31554802 Free PMC article.
-
MHC class II deprivation impairs CD4 T cell motility and responsiveness to antigen-bearing dendritic cells in vivo.Proc Natl Acad Sci U S A. 2007 Apr 24;104(17):7181-6. doi: 10.1073/pnas.0608299104. Epub 2007 Apr 13. Proc Natl Acad Sci U S A. 2007. PMID: 17435166 Free PMC article.
-
Effect of Human Genetic Variability on Gene Expression in Dorsal Root Ganglia and Association with Pain Phenotypes.Cell Rep. 2017 May 30;19(9):1940-1952. doi: 10.1016/j.celrep.2017.05.018. Cell Rep. 2017. PMID: 28564610 Free PMC article.
References
-
- Grusby M J, Johnson R S, Papaioannou V E, Glimcher L H. Science. 1991;253:1417–1420. - PubMed
-
- Cosgrove D, Gray D, Dierich A, Kaufman J, Lemeur M, Benoist C, Mathis D. Cell. 1991;66:1051–1066. - PubMed
-
- Köntgen F, Süss G, Stewart C, Steinmetz M, Bluethmann H. Int Immunol. 1993;5:957–964. - PubMed
-
- Mach B, Steimle V, Martinez-Soria E, Reith W. Annu Rev Immunol. 1996;14:301–331. - PubMed
-
- Nepom G T, Erlich H. Annu Rev Immunol. 1991;9:493–525. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

