Phosphoinositide-AP-2 interactions required for targeting to plasma membrane clathrin-coated pits
- PMID: 10459011
- PMCID: PMC2156139
- DOI: 10.1083/jcb.146.4.755
Phosphoinositide-AP-2 interactions required for targeting to plasma membrane clathrin-coated pits
Abstract
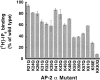
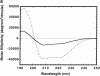
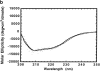
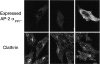
The clathrin-associated AP-2 adaptor protein is a major polyphosphoinositide-binding protein in mammalian cells. A high affinity binding site has previously been localized to the NH(2)-terminal region of the AP-2 alpha subunit (Gaidarov et al. 1996. J. Biol. Chem. 271:20922-20929). Here we used deletion and site- directed mutagenesis to determine that alpha residues 21-80 comprise a discrete folding and inositide-binding domain. Further, positively charged residues located within this region are involved in binding, with a lysine triad at positions 55-57 particularly critical. Mutant peptides and protein in which these residues were changed to glutamine retained wild-type structural and functional characteristics by several criteria including circular dichroism spectra, resistance to limited proteolysis, and clathrin binding activity. When expressed in intact cells, mutated alpha subunit showed defective localization to clathrin-coated pits; at high expression levels, the appearance of endogenous AP-2 in coated pits was also blocked consistent with a dominant-negative phenotype. These results, together with recent work indicating that phosphoinositides are also critical to ligand-dependent recruitment of arrestin-receptor complexes to coated pits (Gaidarov et al. 1999. EMBO (Eur. Mol. Biol. Organ.) J. 18:871-881), suggest that phosphoinositides play a critical and general role in adaptor incorporation into plasma membrane clathrin-coated pits.
Figures
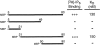



Similar articles
-
The interaction of beta-arrestin with the AP-2 adaptor is required for the clustering of beta 2-adrenergic receptor into clathrin-coated pits.J Biol Chem. 2000 Jul 28;275(30):23120-6. doi: 10.1074/jbc.M002581200. J Biol Chem. 2000. PMID: 10770944
-
Arrestin/clathrin interaction. Localization of the arrestin binding locus to the clathrin terminal domain.J Biol Chem. 1997 Jun 6;272(23):15017-22. doi: 10.1074/jbc.272.23.15017. J Biol Chem. 1997. PMID: 9169477
-
Epidermal growth factor receptor interaction with clathrin adaptors is mediated by the Tyr974-containing internalization motif.J Biol Chem. 1996 Jun 7;271(23):13377-84. doi: 10.1074/jbc.271.23.13377. J Biol Chem. 1996. PMID: 8662849
-
Adaptors for clathrin-mediated traffic.Annu Rev Cell Dev Biol. 1999;15:705-32. doi: 10.1146/annurev.cellbio.15.1.705. Annu Rev Cell Dev Biol. 1999. PMID: 10611976 Review.
-
Assembly of clathrin-coated pits onto purified plasma membranes.Science. 1987 May 1;236(4801):558-63. doi: 10.1126/science.2883727. Science. 1987. PMID: 2883727 Review.
Cited by
-
Effects of wortmannin and latrunculin A on slow endocytosis at the frog neuromuscular junction.J Physiol. 2004 May 15;557(Pt 1):77-91. doi: 10.1113/jphysiol.2004.062158. Epub 2004 Mar 5. J Physiol. 2004. PMID: 15004214 Free PMC article.
-
Clathrin-dependent endocytosis.Biochem J. 2004 Jan 1;377(Pt 1):1-16. doi: 10.1042/BJ20031000. Biochem J. 2004. PMID: 14505490 Free PMC article. Review.
-
Clathrin promotes incorporation of cargo into coated pits by activation of the AP2 adaptor micro2 kinase.J Cell Biol. 2003 Oct 27;163(2):231-6. doi: 10.1083/jcb.200304079. J Cell Biol. 2003. PMID: 14581451 Free PMC article.
-
Peptide motifs: building the clathrin machinery.Mol Neurobiol. 2005 Aug;32(1):73-87. doi: 10.1385/MN:32:1:073. Mol Neurobiol. 2005. PMID: 16077185 Review.
-
Phosphatidylinositol-(4,5)-bisphosphate regulates clathrin-coated pit initiation, stabilization, and size.Mol Biol Cell. 2011 Jul 15;22(14):2588-600. doi: 10.1091/mbc.E11-04-0362. Epub 2011 May 25. Mol Biol Cell. 2011. PMID: 21613550 Free PMC article.
References
-
- Andrade M.A., Chacon P., Merelo J.J., Moran F. Evaluation of secondary structure of proteins from UV circular dichroism spectra using an unsupervised learning neural network. Protein Eng. 1993;6:383–390. - PubMed
-
- Barylko B., Binns D., Lin K., Atkinson M.A., Jameson D.M., Yin H.L., Albanesi J.P. Synergistic activation of dynamin GTPase by Grb2 and phosphoinositides. J. Biol. Chem. 1998;273:3791–3797. - PubMed
-
- Beck K.A., Keen J.H. Interaction of phosphoinositide cycle intermediates with the plasma membrane-associated clathrin assembly protein AP-2. J. Biol. Chem. 1991;266:4442–4447. - PubMed
-
- Benmerah A., Begue B., Dautry-Varsat A., Cerf-Bensussan N. The ear of alpha-adaptin interacts with the COOH-terminal domain of the Eps 15 protein. J. Biol. Chem. 1996;271:12111–12116. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

