Vascular endothelial-cadherin is an important determinant of microvascular integrity in vivo
- PMID: 10449777
- PMCID: PMC22293
- DOI: 10.1073/pnas.96.17.9815
Vascular endothelial-cadherin is an important determinant of microvascular integrity in vivo
Abstract
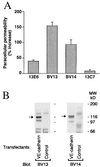
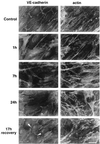
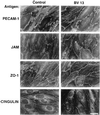
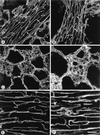
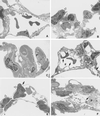
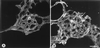
In the present paper, we characterize an antibody, mAb BV13, directed to mouse vascular endothelial (VE)-cadherin, a major adhesive protein of interendothelial adherens junctions. When added to cultured endothelial cells, BV13 induces a redistribution of VE-cadherin from intercellular junctions. VE-cadherin redistribution did not change the localization of platelet endothelial cell adhesion molecule or tight junction markers such as zonula occludens 1, cingulin, and junctional adhesion molecule. Intravenous administration of mAb BV13 induced a concentration- and time-dependent increase in vascular permeability in heart and lungs. By electron microscopy, interstitial edema and accumulation of mixed types of inflammatory cells in heart and lungs were observed. Injection of (rhodamine-labeled) Ricinus communis I lectin showed focal spots of exposed basement membrane in the alveolar capillaries and in some larger pulmonary vessels. These data indicate that VE-cadherin is required for vascular integrity and normal organ functions.
Figures

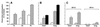
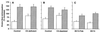

Similar articles
-
A monoclonal antibody to vascular endothelial-cadherin inhibits tumor angiogenesis without side effects on endothelial permeability.Blood. 2002 Aug 1;100(3):905-11. doi: 10.1182/blood.v100.3.905. Blood. 2002. PMID: 12130501
-
Selective targeting of angiogenic tumor vasculature by vascular endothelial-cadherin antibody inhibits tumor growth without affecting vascular permeability.Cancer Res. 2002 May 1;62(9):2567-75. Cancer Res. 2002. PMID: 11980651
-
Interference With ESAM (Endothelial Cell-Selective Adhesion Molecule) Plus Vascular Endothelial-Cadherin Causes Immediate Lethality and Lung-Specific Blood Coagulation.Arterioscler Thromb Vasc Biol. 2020 Feb;40(2):378-393. doi: 10.1161/ATVBAHA.119.313545. Epub 2019 Dec 12. Arterioscler Thromb Vasc Biol. 2020. PMID: 31826650
-
VE-cadherin: the major endothelial adhesion molecule controlling cellular junctions and blood vessel formation.Arterioscler Thromb Vasc Biol. 2008 Feb;28(2):223-32. doi: 10.1161/ATVBAHA.107.158014. Epub 2007 Dec 27. Arterioscler Thromb Vasc Biol. 2008. PMID: 18162609 Review.
-
Vascular endothelial (VE)-cadherin: only an intercellular glue?Exp Cell Res. 1999 Oct 10;252(1):13-9. doi: 10.1006/excr.1999.4601. Exp Cell Res. 1999. PMID: 10502395 Review.
Cited by
-
Adrenomedullin blockade induces regression of tumor neovessels through interference with vascular endothelial-cadherin signalling.Oncotarget. 2015 Apr 10;6(10):7536-53. doi: 10.18632/oncotarget.3167. Oncotarget. 2015. PMID: 25924235 Free PMC article.
-
Auraptene Enhances Junction Assembly in Cerebrovascular Endothelial Cells by Promoting Resilience to Mitochondrial Stress through Activation of Antioxidant Enzymes and mtUPR.Antioxidants (Basel). 2021 Mar 17;10(3):475. doi: 10.3390/antiox10030475. Antioxidants (Basel). 2021. PMID: 33802930 Free PMC article.
-
Pathophysiology in Brain Arteriovenous Malformations: Focus on Endothelial Dysfunctions and Endothelial-to-Mesenchymal Transition.Biomedicines. 2024 Aug 7;12(8):1795. doi: 10.3390/biomedicines12081795. Biomedicines. 2024. PMID: 39200259 Free PMC article. Review.
-
The Role of the Endothelium in HPS Pathogenesis and Potential Therapeutic Approaches.Adv Virol. 2012;2012:467059. doi: 10.1155/2012/467059. Epub 2012 Jun 28. Adv Virol. 2012. PMID: 22811711 Free PMC article.
-
Receptor protein tyrosine phosphatase micro regulates the paracellular pathway in human lung microvascular endothelia.Am J Pathol. 2005 Apr;166(4):1247-58. doi: 10.1016/s0002-9440(10)62343-7. Am J Pathol. 2005. PMID: 15793303 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

