Cdc25B and Cdc25C differ markedly in their properties as initiators of mitosis
- PMID: 10444066
- PMCID: PMC2150562
- DOI: 10.1083/jcb.146.3.573
Cdc25B and Cdc25C differ markedly in their properties as initiators of mitosis
Abstract
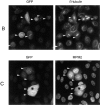
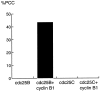
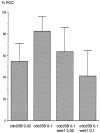
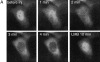
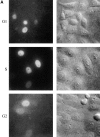
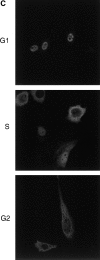
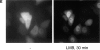
We have used time-lapse fluorescence microscopy to study the properties of the Cdc25B and Cdc25C phosphatases that have both been implicated as initiators of mitosis in human cells. To differentiate between the functions of the two proteins, we have microinjected expression constructs encoding Cdc25B or Cdc25C or their GFP-chimeras into synchronized tissue culture cells. This assay allows us to express the proteins at defined points in the cell cycle. We have followed the microinjected cells by time-lapse microscopy, in the presence or absence of DNA synthesis inhibitors, and assayed whether they enter mitosis prematurely or at the correct time. We find that overexpressing Cdc25B alone rapidly causes S phase and G2 phase cells to enter mitosis, whether or not DNA replication is complete, whereas overexpressing Cdc25C does not cause premature mitosis. Overexpressing Cdc25C together with cyclin B1 does shorten the G2 phase and can override the unreplicated DNA checkpoint, but much less efficiently than overexpressing Cdc25B. These results suggest that Cdc25B and Cdc25C do not respond identically to the same cell cycle checkpoints. This difference may be related to the differential localization of the proteins; Cdc25C is nuclear throughout interphase, whereas Cdc25B is nuclear in the G1 phase and cytoplasmic in the S and G2 phases. We have found that the change in subcellular localization of Cdc25B is due to nuclear export and that this is dependent on cyclin B1. Our data suggest that although both Cdc25B and Cdc25C can promote mitosis, they are likely to have distinct roles in the controlling the initiation of mitosis.
Figures

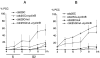





Similar articles
-
Hyperphosphorylation of the N-terminal domain of Cdc25 regulates activity toward cyclin B1/Cdc2 but not cyclin A/Cdk2.J Biol Chem. 1997 Nov 7;272(45):28607-14. doi: 10.1074/jbc.272.45.28607. J Biol Chem. 1997. PMID: 9353326
-
MPF localization is controlled by nuclear export.EMBO J. 1998 Jul 15;17(14):4127-38. doi: 10.1093/emboj/17.14.4127. EMBO J. 1998. PMID: 9670027 Free PMC article.
-
The cdc25B phosphatase is essential for the G2/M phase transition in human cells.J Cell Sci. 1998 Aug;111 ( Pt 16):2445-53. doi: 10.1242/jcs.111.16.2445. J Cell Sci. 1998. PMID: 9683638
-
Control of mitosis by changes in the subcellular location of cyclin-B1-Cdk1 and Cdc25C.Curr Opin Cell Biol. 2000 Dec;12(6):658-65. doi: 10.1016/s0955-0674(00)00149-6. Curr Opin Cell Biol. 2000. PMID: 11063929 Review.
-
Proteasome-dependent degradation of human CDC25B phosphatase.Mol Biol Rep. 1999 Apr;26(1-2):53-7. doi: 10.1023/a:1006912105352. Mol Biol Rep. 1999. PMID: 10363647 Review.
Cited by
-
Ser149 is another potential PKA phosphorylation target of Cdc25B in G2/M transition of fertilized mouse eggs.J Biol Chem. 2011 Mar 25;286(12):10356-66. doi: 10.1074/jbc.M110.150524. Epub 2011 Jan 6. J Biol Chem. 2011. PMID: 21212267 Free PMC article.
-
PAR proteins direct asymmetry of the cell cycle regulators Polo-like kinase and Cdc25.J Cell Biol. 2008 Mar 10;180(5):877-85. doi: 10.1083/jcb.200710018. Epub 2008 Mar 3. J Cell Biol. 2008. PMID: 18316412 Free PMC article.
-
RCC1-dependent activation of Ran accelerates cell cycle and DNA repair, inhibiting DNA damage-induced cell senescence.Mol Biol Cell. 2016 Apr 15;27(8):1346-57. doi: 10.1091/mbc.E16-01-0025. Epub 2016 Feb 10. Mol Biol Cell. 2016. PMID: 26864624 Free PMC article.
-
The Forkhead Box m1b transcription factor is essential for hepatocyte DNA replication and mitosis during mouse liver regeneration.Proc Natl Acad Sci U S A. 2002 Dec 24;99(26):16881-6. doi: 10.1073/pnas.252570299. Epub 2002 Dec 13. Proc Natl Acad Sci U S A. 2002. PMID: 12482952 Free PMC article.
-
The Renaissance or the cuckoo clock.Philos Trans R Soc Lond B Biol Sci. 2011 Dec 27;366(1584):3625-34. doi: 10.1098/rstb.2011.0080. Philos Trans R Soc Lond B Biol Sci. 2011. PMID: 22084388 Free PMC article. Review.
References
-
- Amon A., Surana U., Muroff I., Nasmyth K. Regulation of p34CDC28 tyrosine phosphorylation is not required for entry into mitosis in S. cerevisiae . Nature. 1992;355:368–371. - PubMed
-
- Amon A., Irniger S., Nasmyth K. Closing the cell cycle circle in yeastG2 cyclin proteolysis initiated at mitosis persists until the activation of G1 cyclins in the next cycle. Cell. 1994;77:1037–1050. - PubMed
-
- Baldin V., Cans C., Knibiehler M., Ducommun B. Phosphorylation of human CDC25B phosphatase by CDK1-cyclin A triggers its proteasome-dependent degradation J. Biol. Chem 272 1997. 32731 32734a - PubMed
-
- Baldin V., Cans C., Superti Furga G., Ducommun B. Alternative splicing of the human CDC25B tyrosine phosphatase. Possible implications for growth control? Oncogene 14 1997. 2485 2495b - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

