Clathrin assembly lymphoid myeloid leukemia (CALM) protein: localization in endocytic-coated pits, interactions with clathrin, and the impact of overexpression on clathrin-mediated traffic
- PMID: 10436022
- PMCID: PMC25500
- DOI: 10.1091/mbc.10.8.2687
Clathrin assembly lymphoid myeloid leukemia (CALM) protein: localization in endocytic-coated pits, interactions with clathrin, and the impact of overexpression on clathrin-mediated traffic
Abstract
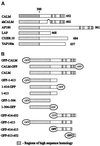
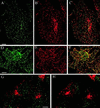
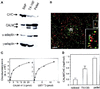
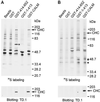
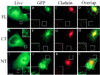
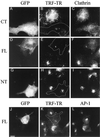
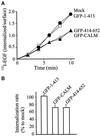
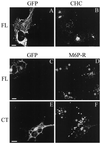
The clathrin assembly lymphoid myeloid leukemia (CALM) gene encodes a putative homologue of the clathrin assembly synaptic protein AP180. Hence the biochemical properties, the subcellular localization, and the role in endocytosis of a CALM protein were studied. In vitro binding and coimmunoprecipitation demonstrated that the clathrin heavy chain is the major binding partner of CALM. The bulk of cellular CALM was associated with the membrane fractions of the cell and localized to clathrin-coated areas of the plasma membrane. In the membrane fraction, CALM was present at near stoichiometric amounts relative to clathrin. To perform structure-function analysis of CALM, we engineered chimeric fusion proteins of CALM and its fragments with the green fluorescent protein (GFP). GFP-CALM was targeted to the plasma membrane-coated pits and also found colocalized with clathrin in the Golgi area. High levels of expression of GFP-CALM or its fragments with clathrin-binding activity inhibited the endocytosis of transferrin and epidermal growth factor receptors and altered the steady-state distribution of the mannose-6-phosphate receptor in the cell. In addition, GFP-CALM overexpression caused the loss of clathrin accumulation in the trans-Golgi network area, whereas the localization of the clathrin adaptor protein complex 1 in the trans-Golgi network remained unaffected. The ability of the GFP-tagged fragments of CALM to affect clathrin-mediated processes correlated with the targeting of the fragments to clathrin-coated areas and their clathrin-binding capacities. Clathrin-CALM interaction seems to be regulated by multiple contact interfaces. The C-terminal part of CALM binds clathrin heavy chain, although the full-length protein exhibited maximal ability for interaction. Altogether, the data suggest that CALM is an important component of coated pit internalization machinery, possibly involved in the regulation of clathrin recruitment to the membrane and/or the formation of the coated pit.
Figures
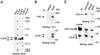

Similar articles
-
Expression of auxilin or AP180 inhibits endocytosis by mislocalizing clathrin: evidence for formation of nascent pits containing AP1 or AP2 but not clathrin.J Cell Sci. 2001 Jan;114(Pt 2):353-65. doi: 10.1242/jcs.114.2.353. J Cell Sci. 2001. PMID: 11148137
-
A conserved clathrin assembly motif essential for synaptic vesicle endocytosis.J Neurosci. 2000 Dec 1;20(23):8667-76. doi: 10.1523/JNEUROSCI.20-23-08667.2000. J Neurosci. 2000. PMID: 11102472 Free PMC article.
-
Simultaneous binding of PtdIns(4,5)P2 and clathrin by AP180 in the nucleation of clathrin lattices on membranes.Science. 2001 Feb 9;291(5506):1051-5. doi: 10.1126/science.291.5506.1051. Science. 2001. PMID: 11161218
-
Clathrin: its role in receptor-mediated vesicular transport and specialized functions in neurons.Crit Rev Biochem Mol Biol. 1993;28(5):431-64. doi: 10.3109/10409239309078441. Crit Rev Biochem Mol Biol. 1993. PMID: 8269710 Review.
-
Cargo recognition during clathrin-mediated endocytosis: a team effort.Curr Opin Cell Biol. 2004 Aug;16(4):392-9. doi: 10.1016/j.ceb.2004.06.001. Curr Opin Cell Biol. 2004. PMID: 15261671 Review.
Cited by
-
PICALM exerts a role in promoting CRC progression through ERK/MAPK signaling pathway.Cancer Cell Int. 2022 May 2;22(1):178. doi: 10.1186/s12935-022-02577-z. Cancer Cell Int. 2022. PMID: 35501863 Free PMC article.
-
Acute myeloid leukemia driven by the CALM-AF10 fusion gene is dependent on BMI1.Exp Hematol. 2019 Jun;74:42-51.e3. doi: 10.1016/j.exphem.2019.04.003. Epub 2019 May 13. Exp Hematol. 2019. PMID: 31022428 Free PMC article.
-
CALM, a clathrin assembly protein, influences cell surface GluR2 abundance.Neuromolecular Med. 2011 Mar;13(1):88-90. doi: 10.1007/s12017-010-8142-6. Epub 2011 Jan 8. Neuromolecular Med. 2011. PMID: 21221849
-
Insight into nanoparticle cellular uptake and intracellular targeting.J Control Release. 2014 Sep 28;190:485-99. doi: 10.1016/j.jconrel.2014.06.038. Epub 2014 Jun 28. J Control Release. 2014. PMID: 24984011 Free PMC article. Review.
-
Replication of CLU, CR1, and PICALM associations with alzheimer disease.Arch Neurol. 2010 Aug;67(8):961-4. doi: 10.1001/archneurol.2010.147. Epub 2010 Jun 14. Arch Neurol. 2010. PMID: 20554627 Free PMC article. Clinical Trial.
References
-
- Chen H, Fre S, Slepnev VI, Capua MR, Takei K, Butler MH, Di Fiore PP, De Camilli P. Epsin is an EH-domain-binding protein implicated in clathrin-mediated endocytosis. Nature. 1998;394:793–797. - PubMed
-
- De Beer T, Carter RE, Lobel-Rice KE, Sorkin A, Overduin M. Structure and Asn-Pro-Phe binding pocket of the eps15 homology domain. Science. 1998;281:1357–1360. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

