Opacity-associated protein A contributes to the binding of Haemophilus influenzae to chang epithelial cells
- PMID: 10417187
- PMCID: PMC96720
- DOI: 10.1128/IAI.67.8.4153-4160.1999
Opacity-associated protein A contributes to the binding of Haemophilus influenzae to chang epithelial cells
Abstract
Opacity-associated protein A (OapA), which is responsible for the transparent-colony phenotype of Haemophilus influenzae, has been implicated in the colonization of the nasopharynx in an infant rat model of carriage. In this report, we show that OapA mediates attachment to Chang epithelial cells examined by using genetically defined type b and nontypeable H. influenzae strains with or without OapA. We also showed that OapA was conserved among H. influenzae strains by comparing deduced amino acid sequences. Both recombinant OapA and polyclonal anti-OapA antiserum blocked the binding of H. influenzae to Chang epithelial cells, suggesting that the interaction of H. influenzae is specific to OapA. Moreover, the binding of recombinant OapA to epithelial cells further provided evidence that OapA can promote attachment of H. influenzae. Expression of oapA gene in a nonadherent Escherichia coli strain significantly increased the binding to Chang epithelial cells, and disruption of the oapA gene with kanamycin resistance cassette insertion resulted in a significant loss of binding. These findings demonstrate that OapA plays a role in H. influenzae binding to human conjunctival epithelial cells.
Figures
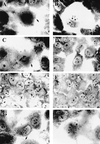


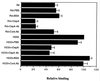

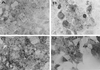
Similar articles
-
Identification and characterization of a cell envelope protein of Haemophilus influenzae contributing to phase variation in colony opacity and nasopharyngeal colonization.Mol Microbiol. 1995 Aug;17(3):555-64. doi: 10.1111/j.1365-2958.1995.mmi_17030555.x. Mol Microbiol. 1995. PMID: 8559074
-
Analysis of the attachment and invasion of human epithelial cells by Haemophilus influenzae biogroup aegyptius.J Infect Dis. 1992 Jun;165 Suppl 1:S111-4. doi: 10.1093/infdis/165-supplement_1-s111. J Infect Dis. 1992. PMID: 1588140 No abstract available.
-
Adherence of Streptococcus pyogenes to human epithelial cells is modulated by Haemophilus influenzae.Scand J Infect Dis. 2009;41(4):244-51. doi: 10.1080/00365540902767064. Scand J Infect Dis. 2009. PMID: 19214868
-
Molecular determinants of the interaction between Haemophilus influenzae and human cells.Am J Respir Crit Care Med. 1996 Oct;154(4 Pt 2):S192-6. doi: 10.1164/ajrccm/154.4_Pt_2.S192. Am J Respir Crit Care Med. 1996. PMID: 8876541 Review.
-
Role of pili in Haemophilus influenzae adherence and colonization.Infect Immun. 1997 Aug;65(8):2997-3002. doi: 10.1128/iai.65.8.2997-3002.1997. Infect Immun. 1997. PMID: 9234745 Free PMC article. Review. No abstract available.
Cited by
-
Transformed Recombinant Enrichment Profiling Rapidly Identifies HMW1 as an Intracellular Invasion Locus in Haemophilus influenza.PLoS Pathog. 2016 Apr 28;12(4):e1005576. doi: 10.1371/journal.ppat.1005576. eCollection 2016 Apr. PLoS Pathog. 2016. PMID: 27124727 Free PMC article.
-
Crystal structure of a putative lysostaphin peptidase from Vibrio cholerae.Proteins. 2008 Aug 15;72(3):1096-103. doi: 10.1002/prot.22095. Proteins. 2008. PMID: 18498110 Free PMC article. No abstract available.
-
The HMW1 and HMW2 Adhesins Enhance the Ability of Nontypeable Haemophilus influenzae To Colonize the Upper Respiratory Tract of Rhesus Macaques.Infect Immun. 2016 Sep 19;84(10):2771-8. doi: 10.1128/IAI.00153-16. Print 2016 Oct. Infect Immun. 2016. PMID: 27430270 Free PMC article.
-
Identification and characterization of a nontypeable Haemophilus influenzae putative toxin-antitoxin locus.BMC Microbiol. 2004 Jul 26;4:30. doi: 10.1186/1471-2180-4-30. BMC Microbiol. 2004. PMID: 15274747 Free PMC article.
-
Complete genome sequence of Haemophilus parasuis SH0165.J Bacteriol. 2009 Feb;191(4):1359-60. doi: 10.1128/JB.01682-08. Epub 2008 Dec 12. J Bacteriol. 2009. PMID: 19074396 Free PMC article.
References
-
- Barenkamp S J, St. Geme J W., III Identification of a second family of high-molecular weight adhesion proteins expressed by nontypeable Haemophilus influenzae. Mol Microbiol. 1996;19:1215–1223. - PubMed
-
- Farley M M, Stephens D S, Kaplan S L, Mason E O., Jr Pilus and non-pilus mediated interactions of Haemophilus influenzae type b with human erythrocytes and human nasopharyngeal mucosa. J Infect Dis. 1990;161:274–280. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

