Stimulation of neonatal and adult brain neurogenesis by subcutaneous injection of basic fibroblast growth factor
- PMID: 10407038
- PMCID: PMC6783097
- DOI: 10.1523/JNEUROSCI.19-14-06006.1999
Stimulation of neonatal and adult brain neurogenesis by subcutaneous injection of basic fibroblast growth factor
Abstract
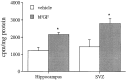
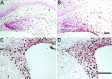
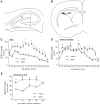
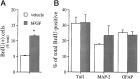
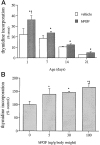
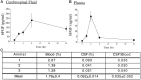
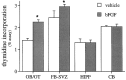
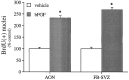
Mounting evidence indicates that extracellular factors exert proliferative effects on neurogenetic precursors in vivo. Recently we found that systemic levels of basic fibroblast growth factor (bFGF) regulate neurogenesis in the brain of newborn rats, with factors apparently crossing the blood-brain barrier (BBB) to stimulate mitosis. To determine whether peripheral bFGF affects proliferation during adulthood, we focused on regions in which neurogenesis persists into maturity, the hippocampus and the forebrain subventricular zone (SVZ). In postnatal day 1 (P1) rats, 8 hr after subcutaneous injection (5 ng/gm body weight), bFGF increased [(3)H]thymidine incorporation 70% in hippocampal and SVZ homogenates and elicited twofold increases in mitotic nuclei in the dentate gyrus and the dorsolateral SVZ, detected by bromodeoxyuridine immunohistochemistry. Because approximately 25% of proliferating hippocampal cells stimulated in vivo expressed neuronal traits in culture, bFGF-induced mitosis may reflect increased neurogenesis. bFGF effects were not restricted to the perinatal period; hippocampal DNA synthesis was stimulated by peripheral factor in older animals (P7-P21), indicating the persistence of bFGF-responsive cells and activity of peripheral bFGF into late development. To begin defining underlying mechanisms, pharmacokinetic studies were performed in P28 rats; bFGF transferred from plasma to CSF rapidly, levels rising in both compartments in parallel, indicating that peripheral factor crosses the BBB during maturity. Consequently, we tested bFGF in adults; peripheral bFGF increased the number of mitotic nuclei threefold in the SVZ and olfactory tract, regions exhibiting persistent neurogenesis. Our observations suggest that bFGF regulates ongoing neurogenesis via a unique, endocrine-like pathway, potentially coordinating neuron number and body growth, and potentially providing new approaches for treating damaged brain during development and adulthood.
Figures


Similar articles
-
Neurogenesis in neonatal rat brain is regulated by peripheral injection of basic fibroblast growth factor (bFGF).J Comp Neurol. 1996 Dec 23;376(4):653-63. doi: 10.1002/(SICI)1096-9861(19961223)376:4<653::AID-CNE11>3.0.CO;2-N. J Comp Neurol. 1996. PMID: 8978476
-
Hippocampal granule neuron production and population size are regulated by levels of bFGF.Eur J Neurosci. 2002 Jan;15(1):3-12. doi: 10.1046/j.0953-816x.2001.01832.x. Eur J Neurosci. 2002. PMID: 11860501
-
A single peripheral injection of basic fibroblast growth factor (bFGF) stimulates granule cell production and increases cerebellar growth in newborn rats.J Neurobiol. 2001 Feb 15;46(3):220-9. doi: 10.1002/1097-4695(20010215)46:3<220::aid-neu1004>3.0.co;2-p. J Neurobiol. 2001. PMID: 11169507
-
Immortalized hypothalamic luteinizing hormone-releasing hormone (LHRH) neurons induce a functional switch in the growth factor responsiveness of astroglia: involvement of basic fibroblast growth factor.Int J Dev Neurosci. 2000 Dec;18(8):743-63. doi: 10.1016/s0736-5748(00)00052-6. Int J Dev Neurosci. 2000. PMID: 11154844
-
The FGF-2/FGFRs neurotrophic system promotes neurogenesis in the adult brain.J Neural Transm (Vienna). 2009 Aug;116(8):995-1005. doi: 10.1007/s00702-009-0207-z. Epub 2009 Mar 17. J Neural Transm (Vienna). 2009. PMID: 19291360 Review.
Cited by
-
Engrailed-2 (En2) deletion produces multiple neurodevelopmental defects in monoamine systems, forebrain structures and neurogenesis and behavior.Hum Mol Genet. 2015 Oct 15;24(20):5805-27. doi: 10.1093/hmg/ddv301. Epub 2015 Jul 28. Hum Mol Genet. 2015. PMID: 26220976 Free PMC article.
-
Endogenous repair signaling after brain injury and complementary bioengineering approaches to enhance neural regeneration.Biomark Insights. 2015 May 12;10(Suppl 1):43-60. doi: 10.4137/BMI.S20062. eCollection 2015. Biomark Insights. 2015. PMID: 25983552 Free PMC article. Review.
-
Intrastriatal transforming growth factor alpha delivery to a model of Parkinson's disease induces proliferation and migration of endogenous adult neural progenitor cells without differentiation into dopaminergic neurons.J Neurosci. 2004 Oct 13;24(41):8924-31. doi: 10.1523/JNEUROSCI.2344-04.2004. J Neurosci. 2004. PMID: 15483111 Free PMC article.
-
Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus.J Neurosci. 2001 Mar 1;21(5):1628-34. doi: 10.1523/JNEUROSCI.21-05-01628.2001. J Neurosci. 2001. PMID: 11222653 Free PMC article.
-
Cerebral ischemia and neuroregeneration.Neural Regen Res. 2018 Mar;13(3):373-385. doi: 10.4103/1673-5374.228711. Neural Regen Res. 2018. PMID: 29623912 Free PMC article. Review.
References
-
- Altman J, Bayer SA. Migration and distribution of two populations of hippocampal granule cell precursors during the perinatal and postnatal period. J Comp Neurol. 1990;301:365–381. - PubMed
-
- Baird A. Fibroblast growth factors: activities and significance of non-neurotrophin neurotrophic growth factors. Curr Opin Neurobiol. 1994;4:78–86. - PubMed
-
- Bethel A, Kirsch JR, Koehler RC, Finklestein SP, Traystman RJ. Intravenous basic fibroblast growth factor decreases brain injury resulting from focal ischemia in cats. Stroke. 1997;28:609–615. - PubMed
-
- Biegler SC, Henkel AW, Unsicker K. Localization of basic fibroblast growth factor in bovine adrenal chromaffin cells. J Neurochem. 1995;64:1521–1527. - PubMed
-
- Bohn MC, Lauder JM. The effects of neonatal hydrocortisone on rat cerebellar development. Dev Neurosci. 1978;1:250–266.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
