Gating kinetics of single large-conductance Ca2+-activated K+ channels in high Ca2+ suggest a two-tiered allosteric gating mechanism
- PMID: 10398695
- PMCID: PMC2229641
- DOI: 10.1085/jgp.114.1.93
Gating kinetics of single large-conductance Ca2+-activated K+ channels in high Ca2+ suggest a two-tiered allosteric gating mechanism
Erratum in
- J Gen Physiol 1999 Aug;114(2):337
Abstract
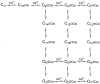
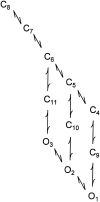
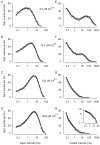
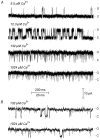
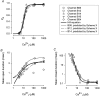
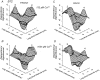
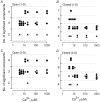
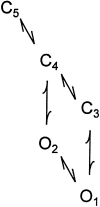
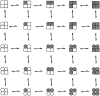
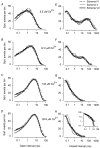
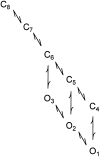
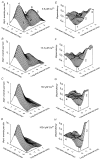
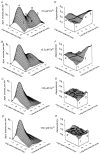
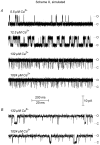
The Ca2+-dependent gating mechanism of large-conductance calcium-activated K+ (BK) channels from cultured rat skeletal muscle was examined from low (4 microM) to high (1,024 microM) intracellular concentrations of calcium (Ca2+i) using single-channel recording. Open probability (Po) increased with increasing Ca2+i (K0. 5 11.2 +/- 0.3 microM at +30 mV, Hill coefficient of 3.5 +/- 0.3), reaching a maximum of approximately 0.97 for Ca2+i approximately 100 microM. Increasing Ca2+i further to 1,024 microM had little additional effect on either Po or the single-channel kinetics. The channels gated among at least three to four open and four to five closed states at high levels of Ca2+i (>100 microM), compared with three to four open and five to seven closed states at lower Ca2+i. The ability of kinetic schemes to account for the single-channel kinetics was examined with simultaneous maximum likelihood fitting of two-dimensional (2-D) dwell-time distributions obtained from low to high Ca2+i. Kinetic schemes drawn from the 10-state Monod-Wyman-Changeux model could not describe the dwell-time distributions from low to high Ca2+i. Kinetic schemes drawn from Eigen's general model for a ligand-activated tetrameric protein could approximate the dwell-time distributions but not the dependency (correlations) between adjacent intervals at high Ca2+i. However, models drawn from a general 50 state two-tiered scheme, in which there were 25 closed states on the upper tier and 25 open states on the lower tier, could approximate both the dwell-time distributions and the dependency from low to high Ca2+i. In the two-tiered model, the BK channel can open directly from each closed state, and a minimum of five open and five closed states are available for gating at any given Ca2+i. A model that assumed that the apparent Ca2+-binding steps can reach a maximum rate at high Ca2+i could also approximate the gating from low to high Ca2+i. The considered models can serve as working hypotheses for the gating of BK channels.
Figures

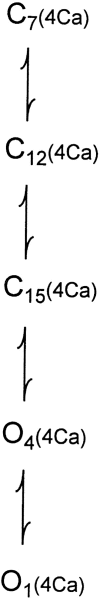


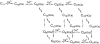




Comment in
-
Commentary: a plausible model.J Gen Physiol. 1999 Aug;114(2):271-5. doi: 10.1085/jgp.114.2.271. J Gen Physiol. 1999. PMID: 10436002 Free PMC article. No abstract available.
Similar articles
-
Single-channel kinetics of BK (Slo1) channels.Front Physiol. 2015 Jan 21;5:532. doi: 10.3389/fphys.2014.00532. eCollection 2014. Front Physiol. 2015. PMID: 25653620 Free PMC article. Review.
-
Voltage and Ca2+ activation of single large-conductance Ca2+-activated K+ channels described by a two-tiered allosteric gating mechanism.J Gen Physiol. 2000 Jul 1;116(1):75-99. doi: 10.1085/jgp.116.1.75. J Gen Physiol. 2000. PMID: 10871641 Free PMC article.
-
Ca2+-dependent gating mechanisms for dSlo, a large-conductance Ca2+-activated K+ (BK) channel.Biophys J. 1999 Jun;76(6):3099-117. doi: 10.1016/S0006-3495(99)77462-X. Biophys J. 1999. PMID: 10354435 Free PMC article.
-
Kinetic structure of large-conductance Ca2+-activated K+ channels suggests that the gating includes transitions through intermediate or secondary states. A mechanism for flickers.J Gen Physiol. 1998 Jun;111(6):751-80. doi: 10.1085/jgp.111.6.751. J Gen Physiol. 1998. PMID: 9607935 Free PMC article.
-
Large conductance Ca2+-activated K+ (BK) channel: activation by Ca2+ and voltage.Biol Res. 2006;39(3):385-401. doi: 10.4067/s0716-97602006000300003. Epub 2006 Nov 7. Biol Res. 2006. PMID: 17106573 Review.
Cited by
-
Differences in Gating Dynamics of BK Channels in Cellular and Mitochondrial Membranes from Human Glioblastoma Cells Unraveled by Short- and Long-Range Correlations Analysis.Cells. 2020 Oct 15;9(10):2305. doi: 10.3390/cells9102305. Cells. 2020. PMID: 33076484 Free PMC article.
-
Slo3 K+ channels: voltage and pH dependence of macroscopic currents.J Gen Physiol. 2006 Sep;128(3):317-36. doi: 10.1085/jgp.200609552. J Gen Physiol. 2006. PMID: 16940555 Free PMC article.
-
Steady-state and closed-state inactivation properties of inactivating BK channels.Biophys J. 2002 May;82(5):2448-65. doi: 10.1016/S0006-3495(02)75588-4. Biophys J. 2002. PMID: 11964233 Free PMC article.
-
Contribution of potential EF hand motifs to the calcium-dependent gating of a mouse brain large conductance, calcium-sensitive K(+) channel.J Physiol. 2001 Jun 15;533(Pt 3):681-95. doi: 10.1111/j.1469-7793.2001.00681.x. J Physiol. 2001. PMID: 11410626 Free PMC article.
-
Single-channel kinetics of BK (Slo1) channels.Front Physiol. 2015 Jan 21;5:532. doi: 10.3389/fphys.2014.00532. eCollection 2014. Front Physiol. 2015. PMID: 25653620 Free PMC article. Review.
References
-
- Adelman J.P., Shen E., Kavanaugh M.P., Warren R.A., Wu Y., Lagrutta A., Bond C., North R.A. Calcium-activated potassium channels expressed from cloned complementary DNAs. Neuron. 1992;9:209–216. - PubMed
-
- Akaike H. A new look at the statistical model identification. IEEE (Inst. Electr. Electron. Eng.) Trans. Auto. Control. 1974;19:716–723.
-
- Atkinson N.S., Robertson G.A., Ganetzky B. A component of calcium-activated potassium channels encoded by the Drosophila slo locus. Science. 1991;253:551–555. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

