The movement of coiled bodies visualized in living plant cells by the green fluorescent protein
- PMID: 10397766
- PMCID: PMC25444
- DOI: 10.1091/mbc.10.7.2297
The movement of coiled bodies visualized in living plant cells by the green fluorescent protein
Abstract
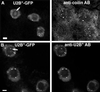
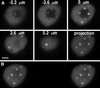
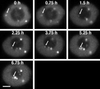
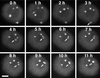
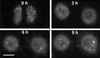
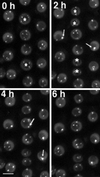
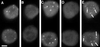
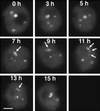
Coiled bodies are nuclear organelles that contain components of at least three RNA-processing pathways: pre-mRNA splicing, histone mRNA 3'- maturation, and pre-rRNA processing. Their function remains unknown. However, it has been speculated that coiled bodies may be sites of splicing factor assembly and/or recycling, play a role in histone mRNA 3'-processing, or act as nuclear transport or sorting structures. To study the dynamics of coiled bodies in living cells, we have stably expressed a U2B"-green fluorescent protein fusion in tobacco BY-2 cells and in Arabidopsis plants. Time-lapse confocal microscopy has shown that coiled bodies are mobile organelles in plant cells. We have observed movements of coiled bodies in the nucleolus, in the nucleoplasm, and from the periphery of the nucleus into the nucleolus, which suggests a transport function for coiled bodies. Furthermore, we have observed coalescence of coiled bodies, which suggests a mechanism for the decrease in coiled body number during the cell cycle. Deletion analysis of the U2B" gene construct has shown that the first RNP-80 motif is sufficient for localization to the coiled body.
Figures

Similar articles
-
Use of fluorescent protein tags to study nuclear organization of the spliceosomal machinery in transiently transformed living plant cells.Mol Biol Cell. 2004 Jul;15(7):3233-43. doi: 10.1091/mbc.e04-01-0055. Epub 2004 May 7. Mol Biol Cell. 2004. PMID: 15133128 Free PMC article.
-
The nucleus: a highly organized but dynamic structure.J Microsc. 2000 Jun;198(Pt 3):199-207. doi: 10.1046/j.1365-2818.2000.00701.x. J Microsc. 2000. PMID: 10849198
-
Dynamic interactions between splicing snRNPs, coiled bodies and nucleoli revealed using snRNP protein fusions to the green fluorescent protein.Exp Cell Res. 1998 Sep 15;243(2):290-304. doi: 10.1006/excr.1998.4135. Exp Cell Res. 1998. PMID: 9743589
-
Cytoplasmic illuminations: in planta targeting of fluorescent proteins to cellular organelles.Protoplasma. 2001;215(1-4):77-88. doi: 10.1007/BF01280305. Protoplasma. 2001. PMID: 11732067 Review.
-
Is the sphere organelle/coiled body a universal nuclear component?Dev Genet. 1995;16(1):25-35. doi: 10.1002/dvg.1020160107. Dev Genet. 1995. PMID: 7758244 Review.
Cited by
-
Nuclear dynamics: Formation of bodies and trafficking in plant nuclei.Front Plant Sci. 2022 Aug 23;13:984163. doi: 10.3389/fpls.2022.984163. eCollection 2022. Front Plant Sci. 2022. PMID: 36082296 Free PMC article. Review.
-
Nuclear bodies and compartmentalization of pre-mRNA splicing factors in higher plants.Chromosoma. 2004 Feb;112(5):255-66. doi: 10.1007/s00412-003-0271-3. Epub 2004 Jan 23. Chromosoma. 2004. PMID: 14740228
-
Use of fluorescent protein tags to study nuclear organization of the spliceosomal machinery in transiently transformed living plant cells.Mol Biol Cell. 2004 Jul;15(7):3233-43. doi: 10.1091/mbc.e04-01-0055. Epub 2004 May 7. Mol Biol Cell. 2004. PMID: 15133128 Free PMC article.
-
Intersection of small RNA pathways in Arabidopsis thaliana sub-nuclear domains.PLoS One. 2013 Jun 12;8(6):e65652. doi: 10.1371/journal.pone.0065652. Print 2013. PLoS One. 2013. PMID: 23776518 Free PMC article.
-
Pearls are novel Cajal body-like structures in the Xenopus germinal vesicle that are dependent on RNA pol III transcription.Chromosome Res. 2012 Dec;20(8):953-69. doi: 10.1007/s10577-012-9320-1. Chromosome Res. 2012. PMID: 23135638 Free PMC article.
References
-
- An G. Binary Ti vectors for plant transformation and promotor analysis. Methods Enzymol. 1987;153:292–305.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

