Recruitment of cyclin T1/P-TEFb to an HIV type 1 long terminal repeat promoter proximal RNA target is both necessary and sufficient for full activation of transcription
- PMID: 10393900
- PMCID: PMC22140
- DOI: 10.1073/pnas.96.14.7791
Recruitment of cyclin T1/P-TEFb to an HIV type 1 long terminal repeat promoter proximal RNA target is both necessary and sufficient for full activation of transcription
Abstract
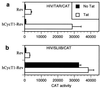
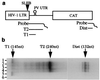
Transcriptional activation of the HIV type 1 (HIV-1) long terminal repeat (LTR) promoter element by the viral Tat protein is an essential step in the HIV-1 life cycle. Tat function is mediated by the TAR RNA target element encoded within the LTR and is known to require the recruitment of a complex consisting of Tat and the cyclin T1 (CycT1) component of positive transcription elongation factor b (P-TEFb) to TAR. Here, we demonstrate that both TAR and Tat become entirely dispensable for activation of the HIV-1 LTR promoter when CycT1/P-TEFb is artificially recruited to a heterologous promoter proximal RNA target. The level of activation observed was indistinguishable from the level induced by Tat and was neither inhibited nor increased when Tat was expressed in trans. Activation by artificially recruited CycT1 depended on the ability to bind the CDK9 component of P-TEFb. In contrast, although binding to both Tat and TAR was essential for the ability of CycT1 to act as a Tat cofactor, these interactions became dispensable when CycT1 was directly recruited to the LTR. Importantly, activation of the LTR both by Tat and by directly recruited CycT1 was found to be at the level of transcription elongation. Together, these data demonstrate that recruitment of CycT1/P-TEFb to the HIV-1 LTR is fully sufficient to activate this promoter element and imply that the sole role of the Tat/TAR axis in viral transcription is to permit the recruitment of CycT1/P-TEFb.
Figures


Similar articles
-
CDK9 autophosphorylation regulates high-affinity binding of the human immunodeficiency virus type 1 tat-P-TEFb complex to TAR RNA.Mol Cell Biol. 2000 Sep;20(18):6958-69. doi: 10.1128/MCB.20.18.6958-6969.2000. Mol Cell Biol. 2000. PMID: 10958691 Free PMC article.
-
Transient induction of cyclin T1 during human macrophage differentiation regulates human immunodeficiency virus type 1 Tat transactivation function.J Virol. 2002 Nov;76(21):10579-87. doi: 10.1128/jvi.76.21.10579-10587.2002. J Virol. 2002. PMID: 12368300 Free PMC article.
-
Analysis of the effect of natural sequence variation in Tat and in cyclin T on the formation and RNA binding properties of Tat-cyclin T complexes.J Virol. 1999 Jul;73(7):5777-86. doi: 10.1128/JVI.73.7.5777-5786.1999. J Virol. 1999. PMID: 10364329 Free PMC article.
-
Regulatory functions of Cdk9 and of cyclin T1 in HIV tat transactivation pathway gene expression.J Cell Biochem. 1999 Dec 1;75(3):357-68. J Cell Biochem. 1999. PMID: 10536359 Review.
-
Tackling Tat.J Mol Biol. 1999 Oct 22;293(2):235-54. doi: 10.1006/jmbi.1999.3060. J Mol Biol. 1999. PMID: 10550206 Review.
Cited by
-
Computational study and peptide inhibitors design for the CDK9 - cyclin T1 complex.J Mol Model. 2013 Apr;19(4):1711-25. doi: 10.1007/s00894-012-1735-2. Epub 2013 Jan 8. J Mol Model. 2013. PMID: 23296566
-
Cross-interaction between JC virus agnoprotein and human immunodeficiency virus type 1 (HIV-1) Tat modulates transcription of the HIV-1 long terminal repeat in glial cells.J Virol. 2006 Sep;80(18):9288-99. doi: 10.1128/JVI.02138-05. J Virol. 2006. PMID: 16940540 Free PMC article.
-
FKBP3 Induces Human Immunodeficiency Virus Type 1 Latency by Recruiting Histone Deacetylase 1/2 to the Viral Long Terminal Repeat.mBio. 2021 Aug 31;12(4):e0079521. doi: 10.1128/mBio.00795-21. Epub 2021 Jul 20. mBio. 2021. PMID: 34281390 Free PMC article.
-
Different modes of enhancer-specific regulation by Runt and Even-skipped during Drosophila segmentation.Mol Biol Cell. 2017 Mar 1;28(5):681-691. doi: 10.1091/mbc.E16-09-0630. Epub 2017 Jan 11. Mol Biol Cell. 2017. PMID: 28077616 Free PMC article.
-
Development of temperature-sensitive mutants of the Drosophila melanogaster P-TEFb (Cyclin T/CDK9) heterodimer using yeast two-hybrid screening.Biochem Biophys Res Commun. 2013 Apr 5;433(2):243-8. doi: 10.1016/j.bbrc.2013.02.091. Epub 2013 Mar 7. Biochem Biophys Res Commun. 2013. PMID: 23500466 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

