Der p 1 facilitates transepithelial allergen delivery by disruption of tight junctions
- PMID: 10393706
- PMCID: PMC408401
- DOI: 10.1172/JCI5844
Der p 1 facilitates transepithelial allergen delivery by disruption of tight junctions
Abstract
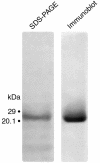
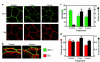
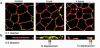
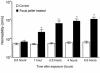
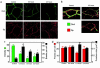
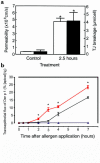
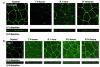
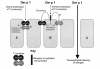
House dust mite (HDM) allergens are important factors in the increasing prevalence of asthma. The lung epithelium forms a barrier that allergens must cross before they can cause sensitization. However, the mechanisms involved are unknown. Here we show that the cysteine proteinase allergen Der p 1 from fecal pellets of the HDM Dermatophagoides pteronyssinus causes disruption of intercellular tight junctions (TJs), which are the principal components of the epithelial paracellular permeability barrier. In confluent airway epithelial cells, Der p 1 led to cleavage of the TJ adhesion protein occludin. Cleavage was attenuated by antipain, but not by inhibitors of serine, aspartic, or matrix metalloproteinases. Putative Der p 1 cleavage sites were found in peptides from an extracellular domain of occludin and in the TJ adhesion protein claudin-1. TJ breakdown nonspecifically increased epithelial permeability, allowing Der p 1 to cross the epithelial barrier. Thus, transepithelial movement of Der p 1 to dendritic antigen-presenting cells via the paracellular pathway may be promoted by the allergen's own proteolytic activity. These results suggest that opening of TJs by environmental proteinases may be the initial step in the development of asthma to a variety of allergens.
Figures



Comment in
-
Understanding tight junction clinical physiology at the molecular level.J Clin Invest. 1999 Jul;104(1):3-4. doi: 10.1172/JCI7599. J Clin Invest. 1999. PMID: 10393692 Free PMC article. No abstract available.
Similar articles
-
The transmembrane protein occludin of epithelial tight junctions is a functional target for serine peptidases from faecal pellets of Dermatophagoides pteronyssinus.Clin Exp Allergy. 2001 Feb;31(2):279-94. doi: 10.1046/j.1365-2222.2001.00970.x. Clin Exp Allergy. 2001. PMID: 11251630
-
Quantitative structural and biochemical analyses of tight junction dynamics following exposure of epithelial cells to house dust mite allergen Der p 1.Clin Exp Allergy. 2000 May;30(5):685-98. doi: 10.1046/j.1365-2222.2000.00820.x. Clin Exp Allergy. 2000. PMID: 10792361
-
Augmentation of permeability in the bronchial epithelium by the house dust mite allergen Der p1.Am J Respir Cell Mol Biol. 1995 Apr;12(4):369-78. doi: 10.1165/ajrcmb.12.4.7695916. Am J Respir Cell Mol Biol. 1995. PMID: 7695916
-
[Mites and their allergens: identification and extermination methods].Allerg Immunol (Paris). 2001 Oct;33(8):333-5. Allerg Immunol (Paris). 2001. PMID: 11763727 Review. French.
-
Allergen-induced generation of mediators in the mucosa.Environ Health Perspect. 2001 Aug;109 Suppl 4(Suppl 4):553-7. doi: 10.1289/ehp.01109s4553. Environ Health Perspect. 2001. PMID: 11544162 Free PMC article. Review.
Cited by
-
Protease and protease-activated receptor-2 signaling in the pathogenesis of atopic dermatitis.Yonsei Med J. 2010 Nov;51(6):808-22. doi: 10.3349/ymj.2010.51.6.808. Yonsei Med J. 2010. PMID: 20879045 Free PMC article. Review.
-
Basal cells of differentiated bronchial epithelium are more susceptible to rhinovirus infection.Am J Respir Cell Mol Biol. 2008 May;38(5):517-23. doi: 10.1165/rcmb.2007-0050OC. Epub 2007 Dec 6. Am J Respir Cell Mol Biol. 2008. PMID: 18063839 Free PMC article.
-
Allergen Delivery Inhibitors: Characterisation of Potent and Selective Inhibitors of Der p 1 and Their Attenuation of Airway Responses to House Dust Mite Allergens.Int J Mol Sci. 2018 Oct 15;19(10):3166. doi: 10.3390/ijms19103166. Int J Mol Sci. 2018. PMID: 30326568 Free PMC article.
-
Barrier Impairment and Type 2 Inflammation in Allergic Diseases: The Pediatric Perspective.Children (Basel). 2021 Dec 9;8(12):1165. doi: 10.3390/children8121165. Children (Basel). 2021. PMID: 34943362 Free PMC article. Review.
-
Protease-activated receptor 2 activation of myeloid dendritic cells regulates allergic airway inflammation.Respir Res. 2011 Sep 21;12(1):122. doi: 10.1186/1465-9921-12-122. Respir Res. 2011. PMID: 21936897 Free PMC article.
References
-
- Tovey ER, Chapman MD, Platts-Mills TAE. Mite faeces are a major source of house dust allergens. Nature. 1981;289:592–593. - PubMed
-
- Sporik R, Holgate ST, Platts-Mills TA, Cogswell JJ. Exposure to house dust mite allergen (Der p 1) and the development of asthma in childhood. A prospective study. N Engl J Med. 1990;323:502–507. - PubMed
-
- Platts-Mills TA, Thomas WR, Aalberse RC, Vervolet D, Chapman MD. Dust mite allergens and asthma: report of a second international workshop. J Allergy Clin Immunol. 1992;89:1046–1060. - PubMed
-
- Peat JK, et al. House dust mite allergens. A major risk factor for childhood asthma in Australia. Am J Respir Crit Care Med. 1996;153:141–146. - PubMed
-
- Dowse GK, Turner KJ, Stewart GA, Alpers MP, Woolcock AJ. The association between Dermatophagoides mites and the increasing prevalence of asthma in village communities within the Papua New Guinea highlands. J Allergy Clin Immunol. 1985;75:75–83. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

