Isolation, cloning, and localization of rat PV-1, a novel endothelial caveolar protein
- PMID: 10366592
- PMCID: PMC2133139
- DOI: 10.1083/jcb.145.6.1189
Isolation, cloning, and localization of rat PV-1, a novel endothelial caveolar protein
Abstract
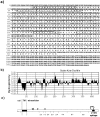
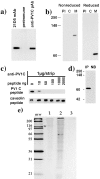
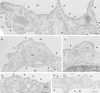
By using an immunoisolation procedure (Stan, R.-V., W.G. Roberts, K. Ihida, D. Predescu, L. Saucan, L. Ghitescu, and G.E. Palade. 1997. Mol. Biol. Cell. 8:595-605) developed in our laboratory, we have isolated a caveolar subfraction from rat lung endothelium and we have partially characterized the proteins of this subfraction which include an apparently caveolae-specific glycoprotein we propose to call PV-1 (formerly known as gp68). The isolation and partial sequencing of PV-1, combined with the cloning of the full length PV-1 cDNA led to the following conclusions: (a) PV-1 is a novel single span type II integral membrane protein (438 amino acids long) which forms homodimers in situ; (b) the transmembrane domain of PV-1 is near the NH2 terminus defining a short cytoplasmic endodomain and a large COOH-terminal ectodomain exposed to the blood plasma; (c) PV-1 is N-glycosylated and its glycan antennae bear terminal nonreducing galactosyl residues in alpha1-3 linkage. PV-1 is expressed mostly in the lung but both the messenger RNA and the protein can be detected at lower levels also in kidney, spleen, liver, heart, muscle, and brain. No signal could be detected in testis and two lower molecular weight forms were detected in brain. Immunocytochemical studies carried out by immunodiffusion on rat lung with an anti-PV-1 polyclonal antibody directed against a COOH-terminal epitope reveal a specific localization of PV-1 to the stomatal diaphragms of rat lung endothelial caveolae and confirm the extracellular orientation of the PV-1 COOH terminus.
Figures



Similar articles
-
PV-1 is a component of the fenestral and stomatal diaphragms in fenestrated endothelia.Proc Natl Acad Sci U S A. 1999 Nov 9;96(23):13203-7. doi: 10.1073/pnas.96.23.13203. Proc Natl Acad Sci U S A. 1999. PMID: 10557298 Free PMC article.
-
Identification and characterization of a major lysosomal membrane glycoprotein, LGP85/LIMP II in mouse liver.J Biochem. 1997 Oct;122(4):756-63. doi: 10.1093/oxfordjournals.jbchem.a021820. J Biochem. 1997. PMID: 9399579
-
Immunoisolation and partial characterization of endothelial plasmalemmal vesicles (caveolae).Mol Biol Cell. 1997 Apr;8(4):595-605. doi: 10.1091/mbc.8.4.595. Mol Biol Cell. 1997. PMID: 9247641 Free PMC article.
-
Molecular characterization and in situ localization of murine endoglin reveal that it is a transforming growth factor-beta binding protein of endothelial and stromal cells.Endocrinology. 1994 Jun;134(6):2645-57. doi: 10.1210/endo.134.6.8194490. Endocrinology. 1994. PMID: 8194490
-
Caveolae: static inpocketings of the plasma membrane, dynamic vesicles or plain artifact?J Cell Sci. 1988 Jul;90 ( Pt 3):341-8. doi: 10.1242/jcs.90.3.341. J Cell Sci. 1988. PMID: 3075612 Review. No abstract available.
Cited by
-
Formation of fenestrae in murine liver sinusoids depends on plasmalemma vesicle-associated protein and is required for lipoprotein passage.PLoS One. 2014 Dec 26;9(12):e115005. doi: 10.1371/journal.pone.0115005. eCollection 2014. PLoS One. 2014. PMID: 25541982 Free PMC article.
-
Caveolae, fenestrae and transendothelial channels retain PV1 on the surface of endothelial cells.PLoS One. 2012;7(3):e32655. doi: 10.1371/journal.pone.0032655. Epub 2012 Mar 5. PLoS One. 2012. PMID: 22403691 Free PMC article.
-
The diaphragms of fenestrated endothelia: gatekeepers of vascular permeability and blood composition.Dev Cell. 2012 Dec 11;23(6):1203-18. doi: 10.1016/j.devcel.2012.11.003. Dev Cell. 2012. PMID: 23237953 Free PMC article.
-
Fenestral diaphragms and PLVAP associations in liver sinusoidal endothelial cells are developmentally regulated.Sci Rep. 2019 Oct 30;9(1):15698. doi: 10.1038/s41598-019-52068-x. Sci Rep. 2019. PMID: 31666588 Free PMC article.
-
Three-Dimensional Architecture of Glomerular Endothelial Cells Revealed by FIB-SEM Tomography.Front Cell Dev Biol. 2021 Mar 11;9:653472. doi: 10.3389/fcell.2021.653472. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33777962 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

