Cold-induced freezing tolerance in Arabidopsis
- PMID: 10364390
- PMCID: PMC59277
- DOI: 10.1104/pp.120.2.391
Cold-induced freezing tolerance in Arabidopsis
Abstract
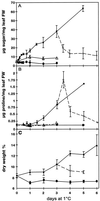
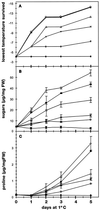
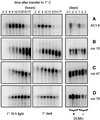
Changes in the physiology of plant leaves are correlated with enhanced freezing tolerance and include accumulation of compatible solutes, changes in membrane composition and behavior, and altered gene expression. Some of these changes are required for enhanced freezing tolerance, whereas others are merely consequences of low temperature. In this study we demonstrated that a combination of cold and light is required for enhanced freezing tolerance in Arabidopsis leaves, and this combination is associated with the accumulation of soluble sugars and proline. Sugar accumulation was evident within 2 h after a shift to low temperature, which preceded measured changes in freezing tolerance. In contrast, significant freezing tolerance was attained before the accumulation of proline or major changes in the percentage of dry weight were detected. Many mRNAs also rapidly accumulated in response to low temperature. All of the cold-induced mRNAs that we examined accumulated at low temperature even in the absence of light, when there was no enhancement of freezing tolerance. Thus, the accumulation of these mRNAs is insufficient for cold-induced freezing tolerance.
Figures

Similar articles
-
Clinal variation in the non-acclimated and cold-acclimated freezing tolerance of Arabidopsis thaliana accessions.Plant Cell Environ. 2012 Oct;35(10):1860-78. doi: 10.1111/j.1365-3040.2012.02522.x. Epub 2012 May 10. Plant Cell Environ. 2012. PMID: 22512351
-
The role of inorganic phosphate in the development of freezing tolerance and the acclimatization of photosynthesis to low temperature is revealed by the pho mutants of Arabidopsis thaliana.Plant J. 2000 Nov;24(3):383-96. doi: 10.1046/j.1365-313x.2000.00888.x. Plant J. 2000. PMID: 11069711
-
Natural genetic variation in acclimation capacity at sub-zero temperatures after cold acclimation at 4 degrees C in different Arabidopsis thaliana accessions.Cryobiology. 2008 Oct;57(2):104-12. doi: 10.1016/j.cryobiol.2008.06.004. Epub 2008 Jun 21. Cryobiology. 2008. PMID: 18619434
-
Interplay between cold-responsive gene regulation, metabolism and RNA processing during plant cold acclimation.Curr Opin Plant Biol. 2007 Jun;10(3):290-5. doi: 10.1016/j.pbi.2007.04.010. Epub 2007 Apr 30. Curr Opin Plant Biol. 2007. PMID: 17468037 Review.
-
[Arabidopsis CBF1 in plant tolerance to low temperature and drought stresses].Yi Chuan. 2004 May;26(3):394-8. Yi Chuan. 2004. PMID: 15640027 Review. Chinese.
Cited by
-
Genetic basis and trade-offs of cold acclimation.Proc Natl Acad Sci U S A. 2024 Mar 5;121(10):e2400501121. doi: 10.1073/pnas.2400501121. Epub 2024 Feb 21. Proc Natl Acad Sci U S A. 2024. PMID: 38381781 Free PMC article. No abstract available.
-
Physiological and Molecular Mechanism of Nitric Oxide (NO) Involved in Bermudagrass Response to Cold Stress.PLoS One. 2015 Jul 15;10(7):e0132991. doi: 10.1371/journal.pone.0132991. eCollection 2015. PLoS One. 2015. PMID: 26177459 Free PMC article.
-
Monitoring expression profiles of Arabidopsis genes during cold acclimation and deacclimation using DNA microarrays.Funct Integr Genomics. 2006 Jul;6(3):212-34. doi: 10.1007/s10142-005-0014-z. Epub 2006 Feb 4. Funct Integr Genomics. 2006. PMID: 16463051
-
Genotype-dependent contribution of CBF transcription factors to long-term acclimation to high light and cool temperature.Plant Cell Environ. 2022 Feb;45(2):392-411. doi: 10.1111/pce.14231. Epub 2021 Dec 6. Plant Cell Environ. 2022. PMID: 34799867 Free PMC article.
-
Association of sugar content QTL and PQL with physiological traits relevant to frost damage resistance in pea under field and controlled conditions.Theor Appl Genet. 2009 May;118(8):1561-71. doi: 10.1007/s00122-009-1004-7. Epub 2009 Mar 26. Theor Appl Genet. 2009. PMID: 19322559
References
-
- Dörffling K, Dörffling H, Lesselich G, Luck E, Zimmermann C, Melz G, Jürgens HU. Heritable improvement of frost tolerance in winter wheat by in-vitro-selection of hydroxy-proline-resistant proline overproducing mutants. Euphytica. 1997;93:1–10.
-
- Dunn MA, Goddard NJ, Zhang L, Pearce RS, Hughes MA. Low-temperature-responsive barley genes have different control mechanisms. Plant Mol Biol. 1994;24:879–888. - PubMed
-
- Farrar JF. Carbon partitioning. In: Hall DO, Scurlock JMO, Bolhár-Nordenkampf HR, Leegood RC, Long SP, editors. Photosynthesis and Production in a Changing Environment: A Field and Laboratory Manual. London: Chapman & Hall; 1993. pp. 232–246.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

