Vesicle-associated membrane protein 4 is implicated in trans-Golgi network vesicle trafficking
- PMID: 10359608
- PMCID: PMC25394
- DOI: 10.1091/mbc.10.6.1957
Vesicle-associated membrane protein 4 is implicated in trans-Golgi network vesicle trafficking
Abstract
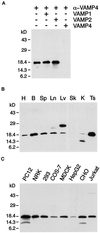
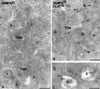
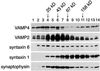
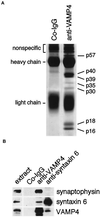
The trans-Golgi network (TGN) plays a pivotal role in directing proteins in the secretory pathway to the appropriate cellular destination. VAMP4, a recently discovered member of the vesicle-associated membrane protein (VAMP) family of trafficking proteins, has been suggested to play a role in mediating TGN trafficking. To better understand the function of VAMP4, we examined its precise subcellular distribution. Indirect immunofluorescence and electron microscopy revealed that the majority of VAMP4 localized to tubular and vesicular membranes of the TGN, which were in part coated with clathrin. In these compartments, VAMP4 was found to colocalize with the putative TGN-trafficking protein syntaxin 6. Additional labeling was also present on clathrin-coated and noncoated vesicles, on endosomes and the medial and trans side of the Golgi complex, as well as on immature secretory granules in PC12 cells. Immunoprecipitation of VAMP4 from rat brain detergent extracts revealed that VAMP4 exists in a complex containing syntaxin 6. Converging lines of evidence implicate a role for VAMP4 in TGN-to-endosome transport.
Figures





Similar articles
-
Syntaxin 6 functions in trans-Golgi network vesicle trafficking.Mol Biol Cell. 1997 Jul;8(7):1261-71. doi: 10.1091/mbc.8.7.1261. Mol Biol Cell. 1997. PMID: 9243506 Free PMC article.
-
VAMP4 is required to maintain the ribbon structure of the Golgi apparatus.Mol Cell Biochem. 2013 Aug;380(1-2):11-21. doi: 10.1007/s11010-013-1652-4. Epub 2013 May 16. Mol Cell Biochem. 2013. PMID: 23677696 Free PMC article.
-
Syntaxin 13 mediates cycling of plasma membrane proteins via tubulovesicular recycling endosomes.J Cell Biol. 1998 Nov 16;143(4):957-71. doi: 10.1083/jcb.143.4.957. J Cell Biol. 1998. PMID: 9817754 Free PMC article.
-
Coat proteins in intracellular membrane transport.Curr Opin Cell Biol. 1994 Aug;6(4):533-7. doi: 10.1016/0955-0674(94)90073-6. Curr Opin Cell Biol. 1994. PMID: 7986530 Review.
-
Coat proteins and vesicle budding.Science. 1996 Mar 15;271(5255):1526-33. doi: 10.1126/science.271.5255.1526. Science. 1996. PMID: 8599108 Review.
Cited by
-
SNARE-Mediated Exocytosis in Neuronal Development.Front Mol Neurosci. 2020 Aug 7;13:133. doi: 10.3389/fnmol.2020.00133. eCollection 2020. Front Mol Neurosci. 2020. PMID: 32848598 Free PMC article. Review.
-
Analysis of SNARE-mediated membrane fusion using an enzymatic cell fusion assay.J Vis Exp. 2012 Oct 19;(68):4378. doi: 10.3791/4378. J Vis Exp. 2012. PMID: 23117158 Free PMC article.
-
Exocytosis mechanisms underlying insulin release and glucose uptake: conserved roles for Munc18c and syntaxin 4.Am J Physiol Regul Integr Comp Physiol. 2010 Mar;298(3):R517-31. doi: 10.1152/ajpregu.00597.2009. Epub 2010 Jan 6. Am J Physiol Regul Integr Comp Physiol. 2010. PMID: 20053958 Free PMC article. Review.
-
Mapping of R-SNARE function at distinct intracellular GLUT4 trafficking steps in adipocytes.J Cell Biol. 2008 Jan 28;180(2):375-87. doi: 10.1083/jcb.200709108. J Cell Biol. 2008. PMID: 18227281 Free PMC article.
-
Souffle/Spastizin controls secretory vesicle maturation during zebrafish oogenesis.PLoS Genet. 2014 Jun 26;10(6):e1004449. doi: 10.1371/journal.pgen.1004449. eCollection 2014 Jun. PLoS Genet. 2014. PMID: 24967841 Free PMC article.
References
-
- Advani RJ, Bae HR, Bock JB, Chao DS, Doung YC, Prekeris R, Yoo JS, Scheller RH. Seven novel mammalian SNARE proteins localize to distinct membrane compartments. J Biol Chem. 1998;273:10317–10324. - PubMed
-
- Banker GA, Cowan WM. Rat hippocampal neurons in dispersed cell culture. Brain Res. 1977;126:397–442. - PubMed
-
- Bennett MK, Scheller RH. A molecular description of synaptic vesicle membrane trafficking. Annu Rev Biochem. 1994;63:63–100. - PubMed
-
- Bock JB, Lin RC, Scheller RH. A new syntaxin family member implicated in targeting of intracellular transport vesicles. J Biol Chem. 1996;271:17961–17965. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

