Phosphorylated RNA polymerase II stimulates pre-mRNA splicing
- PMID: 10346811
- PMCID: PMC316731
- DOI: 10.1101/gad.13.10.1234
Phosphorylated RNA polymerase II stimulates pre-mRNA splicing
Abstract
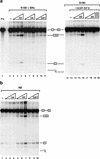
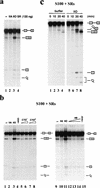
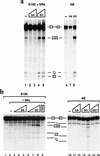
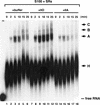
RNA polymerase II (RNAP II) is responsible for transcription of mRNA precursors in eukaryotic cells. Recent studies, however, have suggested that RNAP II also participates in subsequent RNA processing reactions through interactions between the carboxy-terminal domain (CTD) of the RNAP II largest subunit and processing factors. Using reconstituted in vitro splicing assays, we show that RNAP II functions directly in pre-mRNA splicing by influencing very early steps in assembly of the spliceosome. We demonstrate that the phosphorylation status of the CTD dramatically affects activity: Hyperphosphorylated RNAP IIO strongly activates splicing, whereas hypophosphorylated RNAP IIA can inhibit the reaction.
Figures

Similar articles
-
Splicing factors associate with hyperphosphorylated RNA polymerase II in the absence of pre-mRNA.J Cell Biol. 1997 Jan 13;136(1):19-28. doi: 10.1083/jcb.136.1.19. J Cell Biol. 1997. PMID: 9008700 Free PMC article.
-
Participation of the C-terminal domain of RNA polymerase II in exon definition during pre-mRNA splicing.Mol Cell Biol. 2000 Nov;20(21):8290-301. doi: 10.1128/MCB.20.21.8290-8301.2000. Mol Cell Biol. 2000. PMID: 11027297 Free PMC article.
-
Pin1 modulates the structure and function of human RNA polymerase II.Genes Dev. 2003 Nov 15;17(22):2765-76. doi: 10.1101/gad.1135503. Epub 2003 Nov 4. Genes Dev. 2003. PMID: 14600023 Free PMC article.
-
RNA polymerase II conducts a symphony of pre-mRNA processing activities.Biochim Biophys Acta. 2002 Sep 13;1577(2):308-24. doi: 10.1016/s0167-4781(02)00460-8. Biochim Biophys Acta. 2002. PMID: 12213660 Review.
-
Coordination between transcription and pre-mRNA processing.FEBS Lett. 2001 Jun 8;498(2-3):179-82. doi: 10.1016/s0014-5793(01)02485-1. FEBS Lett. 2001. PMID: 11412852 Review.
Cited by
-
Rates of in situ transcription and splicing in large human genes.Nat Struct Mol Biol. 2009 Nov;16(11):1128-33. doi: 10.1038/nsmb.1666. Epub 2009 Oct 11. Nat Struct Mol Biol. 2009. PMID: 19820712 Free PMC article.
-
Regulation of SR protein localization during development.Proc Natl Acad Sci U S A. 2001 Aug 28;98(18):10184-9. doi: 10.1073/pnas.181340498. Proc Natl Acad Sci U S A. 2001. PMID: 11526235 Free PMC article.
-
Smicl is required for phosphorylation of RNA polymerase II and affects 3'-end processing of RNA at the midblastula transition in Xenopus.Development. 2009 Oct;136(20):3451-61. doi: 10.1242/dev.027714. Development. 2009. PMID: 19783735 Free PMC article.
-
Genetic evidence supports a role for the yeast CCR4-NOT complex in transcriptional elongation.Genetics. 2001 Jun;158(2):627-34. doi: 10.1093/genetics/158.2.627. Genetics. 2001. PMID: 11404327 Free PMC article.
-
Intron-Mediated Enhancement: A Tool for Heterologous Gene Expression in Plants?Front Plant Sci. 2017 Jan 6;7:1977. doi: 10.3389/fpls.2016.01977. eCollection 2016. Front Plant Sci. 2017. PMID: 28111580 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
