Conformational intermediates and fusion activity of influenza virus hemagglutinin
- PMID: 10233915
- PMCID: PMC112497
- DOI: 10.1128/JVI.73.6.4567-4574.1999
Conformational intermediates and fusion activity of influenza virus hemagglutinin
Abstract
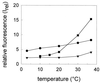
Three strains of influenza virus (H1, H2, and H3) exhibited similar characteristics in the ability of their hemagglutinin (HA) to induce membrane fusion, but the HAs differed in their susceptibility to inactivation. The extent of inactivation depended on the pH of preincubation and was lowest for A/Japan (H2 subtype), in agreement with previous studies (A. Puri, F. Booy, R. W. Doms, J. M. White, and R. Blumenthal, J. Virol. 64:3824-3832, 1990). While significant inactivation of X31 (H3 subtype) was observed at 37 degrees C at pH values corresponding to the maximum of fusion (about pH 5.0), no inactivation was seen at preincubation pH values 0.2 to 0.4 pH units higher. Surprisingly, low-pH preincubation under those conditions enhanced the fusion rates and extents of A/Japan as well as those of X31. For A/PR 8/34 (H1 subtype), neither a shift of the pH (to >5.0) nor a decrease of the temperature to 20 degrees C was sufficient to prevent inactivation. We provide evidence that the activated HA is a conformational intermediate distinct from the native structure and from the final structure associated with the conformational change of HA, which is implicated by the high-resolution structure of the soluble trimeric fragment TBHA2 (P. A. Bullough, F. M. Hughson, J. J. Skehel, and D. C. Wiley, Nature 371:37-43, 1994).
Figures
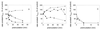

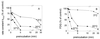
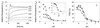

Similar articles
-
Effects of exposure to low pH on the lateral mobility of influenza hemagglutinin expressed at the cell surface: correlation between mobility inhibition and inactivation.Biochemistry. 1993 Jan 12;32(1):101-6. doi: 10.1021/bi00052a014. Biochemistry. 1993. PMID: 8418830
-
Intermediates in influenza virus PR/8 haemagglutinin-induced membrane fusion.J Gen Virol. 1994 Feb;75 ( Pt 2):395-9. doi: 10.1099/0022-1317-75-2-395. J Gen Virol. 1994. PMID: 8113761
-
Conformational changes and fusion activity of influenza virus hemagglutinin of the H2 and H3 subtypes: effects of acid pretreatment.J Virol. 1990 Aug;64(8):3824-32. doi: 10.1128/JVI.64.8.3824-3832.1990. J Virol. 1990. PMID: 2196382 Free PMC article.
-
Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin.Annu Rev Biochem. 2000;69:531-69. doi: 10.1146/annurev.biochem.69.1.531. Annu Rev Biochem. 2000. PMID: 10966468 Review.
-
Early steps of the conformational change of influenza virus hemagglutinin to a fusion active state: stability and energetics of the hemagglutinin.Biochim Biophys Acta. 2003 Jul 11;1614(1):3-13. doi: 10.1016/s0005-2736(03)00158-5. Biochim Biophys Acta. 2003. PMID: 12873761 Review.
Cited by
-
Minimal aggregate size and minimal fusion unit for the first fusion pore of influenza hemagglutinin-mediated membrane fusion.Biophys J. 2000 Jan;78(1):227-45. doi: 10.1016/S0006-3495(00)76587-8. Biophys J. 2000. PMID: 10620288 Free PMC article.
-
Reversible stages of the low-pH-triggered conformational change in influenza virus hemagglutinin.EMBO J. 2002 Nov 1;21(21):5701-10. doi: 10.1093/emboj/cdf559. EMBO J. 2002. PMID: 12411488 Free PMC article.
-
Structural intermediates in the low pH-induced transition of influenza hemagglutinin.PLoS Pathog. 2020 Nov 30;16(11):e1009062. doi: 10.1371/journal.ppat.1009062. eCollection 2020 Nov. PLoS Pathog. 2020. PMID: 33253316 Free PMC article.
-
Structural changes in Influenza virus at low pH characterized by cryo-electron tomography.J Virol. 2012 Mar;86(6):2919-29. doi: 10.1128/JVI.06698-11. Epub 2012 Jan 18. J Virol. 2012. PMID: 22258245 Free PMC article.
-
At low pH, influenza virus matrix protein M1 undergoes a conformational change prior to dissociating from the membrane.J Virol. 2013 May;87(10):5621-8. doi: 10.1128/JVI.00276-13. Epub 2013 Mar 6. J Virol. 2013. PMID: 23468509 Free PMC article.
References
-
- Allan J S, Strauss J, Buck D W. Enhancement of SIV infection with soluble receptor molecules. Science. 1990;247:1084–1088. - PubMed
-
- Arbuzova A, Korte T, Müller P, Herrmann A. On the validity of lipid dequenching assays for estimating virus fusion kinetics. Biochim Biophys Acta. 1994;1190:360–366. - PubMed
-
- Blumenthal R, Schoch C, Puri A, Clague M J. A dissection of steps leading to viral envelope protein-mediated membrane fusion. Ann N Y Acad Sci. 1991;635:285–296. - PubMed
-
- Blumenthal R, Bali-Puri A, Walter A, Covell D, Eidelman O. pH-dependent fusion of vesicular stomatitis virus with Vero cells: measurement by dequenching of octadecyl-rhodamine fluorescence. J Biol Chem. 1987;262:13614–13619. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

