Control of the nuclear localization of Extradenticle by competing nuclear import and export signals
- PMID: 10215621
- PMCID: PMC316638
- DOI: 10.1101/gad.13.8.935
Control of the nuclear localization of Extradenticle by competing nuclear import and export signals
Abstract
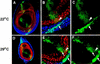
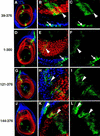
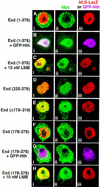
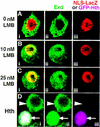
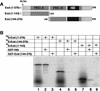
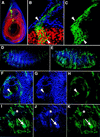
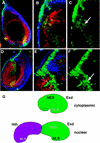
The Drosophila PBC protein Extradenticle (Exd) is regulated at the level of its subcellular distribution: It is cytoplasmic in the absence of Homothorax (Hth), a Meis family member, and nuclear in the presence of Hth. Here we present evidence that, in the absence of Hth, Exd is exported from nuclei due to the activity of a nuclear export signal (NES). The activity of this NES is inhibited by the antibiotic Leptomycin B, suggesting that Exd is exported by a CRM1/exportin1-related export pathway. By analyzing the subcellular localization of Exd deletion mutants in imaginal discs and cultured cells, we identified three elements in Exd, a putative NES, a nuclear localization sequence (NLS), and a region required for Hth-mediated nuclear localization. This latter region coincides with a domain in Exd that binds Hth protein in vitro. When Exd is uncomplexed with Hth, the NES dominates over the NLS. When Exd is expressed together with Hth, or when the NES is deleted, Exd is nuclear. Thus, Hth is required to overcome the influence of the NES, possibly by inducing a conformational change in Exd. Finally, we provide evidence that Hth and Exd normally interact in the cytoplasm, and that Hth also has an NLS. We propose that in Exd there exists a balance between the activities of an NES and an NLS, and that Hth alters this balance in favor of the NLS.
Figures


Similar articles
-
A balance between two nuclear localization sequences and a nuclear export sequence governs extradenticle subcellular localization.Genetics. 2007 Apr;175(4):1625-36. doi: 10.1534/genetics.106.066449. Epub 2007 Feb 4. Genetics. 2007. PMID: 17277370 Free PMC article.
-
The subcellular localization of PBX1 and EXD proteins depends on nuclear import and export signals and is modulated by association with PREP1 and HTH.Genes Dev. 1999 Apr 15;13(8):946-53. doi: 10.1101/gad.13.8.946. Genes Dev. 1999. PMID: 10215622 Free PMC article.
-
Direct interaction of two homeoproteins, homothorax and extradenticle, is essential for EXD nuclear localization and function.Mech Dev. 2000 Mar 1;91(1-2):279-91. doi: 10.1016/s0925-4773(99)00316-0. Mech Dev. 2000. PMID: 10704852
-
The Homothorax homeoprotein activates the nuclear localization of another homeoprotein, extradenticle, and suppresses eye development in Drosophila.Genes Dev. 1998 Feb 1;12(3):435-46. doi: 10.1101/gad.12.3.435. Genes Dev. 1998. PMID: 9450936 Free PMC article.
-
14-3-3 proteins: regulation of subcellular localization by molecular interference.Cell Signal. 2000 Dec;12(11-12):703-9. doi: 10.1016/s0898-6568(00)00131-5. Cell Signal. 2000. PMID: 11152955 Review.
Cited by
-
The advances of E2A-PBX1 fusion in B-cell acute lymphoblastic Leukaemia.Ann Hematol. 2024 Sep;103(9):3385-3398. doi: 10.1007/s00277-023-05595-7. Epub 2023 Dec 27. Ann Hematol. 2024. PMID: 38148344 Review.
-
PBX and MEIS as non-DNA-binding partners in trimeric complexes with HOX proteins.Mol Cell Biol. 1999 Nov;19(11):7577-88. doi: 10.1128/MCB.19.11.7577. Mol Cell Biol. 1999. PMID: 10523646 Free PMC article.
-
Characterization of PREP2, a paralog of PREP1, which defines a novel sub-family of the MEINOX TALE homeodomain transcription factors.Nucleic Acids Res. 2002 May 1;30(9):2043-51. doi: 10.1093/nar/30.9.2043. Nucleic Acids Res. 2002. PMID: 11972344 Free PMC article.
-
miR-147b-modulated expression of vestigial regulates wing development in the bird cherry-oat aphid Rhopalosiphum padi.BMC Genomics. 2020 Jan 22;21(1):71. doi: 10.1186/s12864-020-6466-7. BMC Genomics. 2020. PMID: 31969125 Free PMC article.
-
Antagonism versus cooperativity with TALE cofactors at the base of the functional diversification of Hox protein function.PLoS Genet. 2013;9(2):e1003252. doi: 10.1371/journal.pgen.1003252. Epub 2013 Feb 7. PLoS Genet. 2013. PMID: 23408901 Free PMC article.
References
-
- Abu-Shaar M, Mann RS. Generation of multiple antagonistic domains along the proximodistal axis during Drosophila leg development. Development. 1998;125:3821–3830. - PubMed
-
- Aspland SE, White RA. Nucleocytoplasmic localisation of extradenticle protein is spatially regulated throughout development in Drosophila. Development. 1997;124:741–747. - PubMed
-
- Blank V, Kourilsky P, Israel A. NF-kappa B and related proteins: Rel/dorsal homologies meet ankyrin-like repeats. Trends Biochem Sci. 1992;17:135–140. - PubMed
-
- Brand A, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. - PubMed
-
- Briggs LJ, Stein D, Goltz J, Corrigan VC, Efthymiadis A, Hubner S, Jans DA. The cAMP-dependent protein kinase site (Ser312) enhances dorsal nuclear import through facilitating nuclear localization sequence/importin interaction. J Biol Chem. 1998;273:22745–22752. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
