Molecular cloning and characterization of apricot fruit polyphenol oxidase
- PMID: 10198084
- PMCID: PMC32010
- DOI: 10.1104/pp.119.4.1261
Molecular cloning and characterization of apricot fruit polyphenol oxidase
Abstract
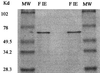
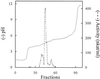
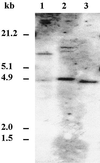
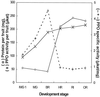
A reverse transcriptase-polymerase chain reaction experiment was done to synthesize a homologous polyphenol oxidase (PPO) probe from apricot (Prunus armeniaca var Bergeron) fruit. This probe was further used to isolate a full-length PPO cDNA, PA-PPO (accession no. AF020786), from an immature-green fruit cDNA library. PA-PPO is 2070 bp long and contains a single open reading frame encoding a PPO precursor peptide of 597 amino acids with a calculated molecular mass of 67.1 kD and an isoelectric point of 6.84. The mature protein has a predicted molecular mass of 56.2 kD and an isoelectric point of 5.84. PA-PPO belongs to a multigene family. The gene is highly expressed in young, immature-green fruit and is turned off early in the ripening process. The ratio of PPO protein to total proteins per fruit apparently remains stable regardless of the stage of development, whereas PPO specific activity peaks at the breaker stage. These results suggest that, in addition to a transcriptional control of PPO expression, other regulation factors such as translational and posttranslational controls also occur.
Figures



Similar articles
-
Molecular cloning and characterisation of banana fruit polyphenol oxidase.Planta. 2001 Sep;213(5):748-57. doi: 10.1007/s004250100553. Planta. 2001. PMID: 11678279
-
An apple polyphenol oxidase cDNA is up-regulated in wounded tissues.Plant Mol Biol. 1995 Jan;27(2):429-33. doi: 10.1007/BF00020197. Plant Mol Biol. 1995. PMID: 7888632
-
Molecular cloning and characterisation of grape berry polyphenol oxidase.Plant Mol Biol. 1994 Oct;26(1):495-502. doi: 10.1007/BF00039560. Plant Mol Biol. 1994. PMID: 7948897
-
Browning in Annona cherimola fruit: role of polyphenol oxidase and characterization of a coding sequence of the enzyme.J Agric Food Chem. 2007 Oct 31;55(22):9208-18. doi: 10.1021/jf070586+. Epub 2007 Oct 2. J Agric Food Chem. 2007. PMID: 17907770
-
Polyphenol oxidase in potato. A multigene family that exhibits differential expression patterns.Plant Physiol. 1995 Oct;109(2):525-31. doi: 10.1104/pp.109.2.525. Plant Physiol. 1995. PMID: 7480344 Free PMC article.
Cited by
-
Polyphenol oxidases in Physcomitrella: functional PPO1 knockout modulates cytokinin-dependent development in the moss Physcomitrella patens.J Exp Bot. 2012 Sep;63(14):5121-35. doi: 10.1093/jxb/ers169. Epub 2012 Aug 3. J Exp Bot. 2012. PMID: 22865913 Free PMC article.
-
Polyphenol oxidase and enzymatic browning in apricot (Prunus armeniaca L.): Effect on phenolic composition and deduction of main substrates.Curr Res Food Sci. 2022 Jan 4;5:196-206. doi: 10.1016/j.crfs.2021.12.015. eCollection 2022. Curr Res Food Sci. 2022. PMID: 35106484 Free PMC article.
-
Allelic variation of polyphenol oxidase (PPO) genes located on chromosomes 2A and 2D and development of functional markers for the PPO genes in common wheat.Theor Appl Genet. 2007 Jun;115(1):47-58. doi: 10.1007/s00122-007-0539-8. Epub 2007 Apr 11. Theor Appl Genet. 2007. PMID: 17426955
-
(+)-Larreatricin hydroxylase, an enantio-specific polyphenol oxidase from the creosote bush (Larrea tridentata).Proc Natl Acad Sci U S A. 2003 Sep 16;100(19):10641-6. doi: 10.1073/pnas.1934562100. Epub 2003 Sep 5. Proc Natl Acad Sci U S A. 2003. PMID: 12960376 Free PMC article.
-
Polyphenol Oxidases in Crops: Biochemical, Physiological and Genetic Aspects.Int J Mol Sci. 2017 Feb 10;18(2):377. doi: 10.3390/ijms18020377. Int J Mol Sci. 2017. PMID: 28208645 Free PMC article.
References
-
- Bernatzky R, Tanksley SD. Genetics of actin-related sequences in tomato. Theor Appl Genet. 1986;72:314–321. - PubMed
-
- Boss PK, Gardner RC, Janssen B-J, Ross GS. An apple polyphenol oxidase cDNA is up-regulated in wounded tissues. Plant Mol Biol. 1995;27:429–433. - PubMed
-
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. - PubMed
-
- Cary JW, Lax AR, Flurkey WH. Cloning and characterization of a cDNA coding for Vicia faba polyphenoloxidase. Plant Mol Biol. 1992;20:245–253. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

