Extraction of cholesterol with methyl-beta-cyclodextrin perturbs formation of clathrin-coated endocytic vesicles
- PMID: 10198050
- PMCID: PMC25220
- DOI: 10.1091/mbc.10.4.961
Extraction of cholesterol with methyl-beta-cyclodextrin perturbs formation of clathrin-coated endocytic vesicles
Abstract
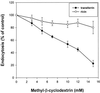
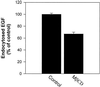
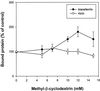
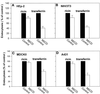
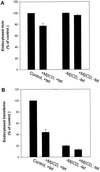
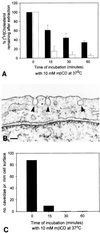
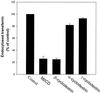
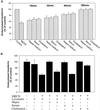
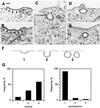
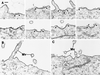
The importance of cholesterol for endocytosis has been investigated in HEp-2 and other cell lines by using methyl-beta-cyclodextrin (MbetaCD) to selectively extract cholesterol from the plasma membrane. MbetaCD treatment strongly inhibited endocytosis of transferrin and EGF, whereas endocytosis of ricin was less affected. The inhibition of transferrin endocytosis was completely reversible. On removal of MbetaCD it was restored by continued incubation of the cells even in serum-free medium. The recovery in serum-free medium was inhibited by addition of lovastatin, which prevents cholesterol synthesis, but endocytosis recovered when a water-soluble form of cholesterol was added together with lovastatin. Electron microscopical studies of MbetaCD-treated HEp-2 cells revealed that typical invaginated caveolae were no longer present. Moreover, the invagination of clathrin-coated pits was strongly inhibited, resulting in accumulation of shallow coated pits. Quantitative immunogold labeling showed that transferrin receptors were concentrated in coated pits to the same degree (approximately sevenfold) after MbetaCD treatment as in control cells. Our results therefore indicate that although clathrin-independent (and caveolae-independent) endocytosis still operates after removal of cholesterol, cholesterol is essential for the formation of clathrin-coated endocytic vesicles.
Figures

Similar articles
-
Regulated endocytosis of G-protein-coupled receptors by a biochemically and functionally distinct subpopulation of clathrin-coated pits.J Biol Chem. 1998 Sep 18;273(38):24592-602. doi: 10.1074/jbc.273.38.24592. J Biol Chem. 1998. PMID: 9733754
-
Transferrin uptake in Trypanosoma cruzi is impaired by interference on cytostome-associated cytoskeleton elements and stability of membrane cholesterol, but not by obstruction of clathrin-dependent endocytosis.Exp Parasitol. 2008 May;119(1):58-66. doi: 10.1016/j.exppara.2007.12.010. Epub 2007 Dec 28. Exp Parasitol. 2008. PMID: 18234197
-
Molecules internalized by clathrin-independent endocytosis are delivered to endosomes containing transferrin receptors.J Cell Biol. 1993 Oct;123(1):89-97. doi: 10.1083/jcb.123.1.89. J Cell Biol. 1993. PMID: 8408209 Free PMC article.
-
Clathrin-dependent or not: is it still the question?Traffic. 2002 Jul;3(7):443-51. doi: 10.1034/j.1600-0854.2002.30701.x. Traffic. 2002. PMID: 12047552 Review.
-
Receptor-mediated endocytosis: the intracellular journey of transferrin and its receptor.Biochimie. 1986 Mar;68(3):375-81. doi: 10.1016/s0300-9084(86)80004-9. Biochimie. 1986. PMID: 2874839 Review.
Cited by
-
Extracellular vesicles derived from macrophages display glycyl-tRNA synthetase 1 and exhibit anti-cancer activity.J Extracell Vesicles. 2020 Nov;10(1):e12029. doi: 10.1002/jev2.12029. Epub 2020 Dec 1. J Extracell Vesicles. 2020. PMID: 33708357 Free PMC article.
-
Arginine topology controls escape of minimally cationic proteins from early endosomes to the cytoplasm.Chem Biol. 2012 Jul 27;19(7):819-30. doi: 10.1016/j.chembiol.2012.05.022. Chem Biol. 2012. PMID: 22840770 Free PMC article.
-
Uptake of ricinB-quantum dot nanoparticles by a macropinocytosis-like mechanism.J Nanobiotechnology. 2012 Jul 31;10:33. doi: 10.1186/1477-3155-10-33. J Nanobiotechnology. 2012. PMID: 22849338 Free PMC article.
-
Cholesterol depletion disorganizes oocyte membrane rafts altering mouse fertilization.PLoS One. 2013 Apr 25;8(4):e62919. doi: 10.1371/journal.pone.0062919. Print 2013. PLoS One. 2013. PMID: 23638166 Free PMC article.
-
Protective role of the dynamin inhibitor Dynasore against the cholesterol-dependent cytolysin of Trueperella pyogenes.FASEB J. 2015 Apr;29(4):1516-28. doi: 10.1096/fj.14-265207. Epub 2014 Dec 30. FASEB J. 2015. PMID: 25550455 Free PMC article.
References
-
- Bloch K. Cholesterol: evolution of structure and function. In: Vance DE, Vance JE, editors. Biochemistry of Lipids, Lipoproteins and Membrane. Amsterdam: Elsevier Science Publishers; 1991. pp. 363–381.
-
- Brown MS, Goldstein JL. Multivalent feedback regulation of HMG CoA reductase, a control mechanism coordinating isoprenoid synthesis and cell growth. J Lipid Res. 1980;21:505–517. - PubMed
-
- Chang JY, Chavis JA, Liu LZ, Drew PD. Cholesterol oxides induce programmed cell death in microglial cells. Biochem Biophys Res Commun. 1998;249:817–821. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

