High resolution detection of mechanical forces exerted by locomoting fibroblasts on the substrate
- PMID: 10198048
- PMCID: PMC25217
- DOI: 10.1091/mbc.10.4.935
High resolution detection of mechanical forces exerted by locomoting fibroblasts on the substrate
Abstract
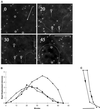
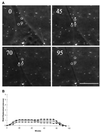
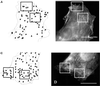
We have developed a new approach to detect mechanical forces exerted by locomoting fibroblasts on the substrate. Cells were cultured on elastic, collagen-coated polyacrylamide sheets embedded with 0. 2-micrometer fluorescent beads. Forces exerted by the cell cause deformation of the substrate and displacement of the beads. By recording the position of beads during cell locomotion and after cell removal, we discovered that most forces were radially distributed, switching direction in the anterior region. Deformations near the leading edge were strong, transient, and variable in magnitude, consistent with active local contractions, whereas those in the posterior region were weaker, more stable, and more uniform, consistent with passive resistance. Treatment of cells with cytochalasin D or myosin II inhibitors caused relaxation of the forces, suggesting that they are generated primarily via actin-myosin II interactions; treatment with nocodazole caused no immediate effect on forces. Immunofluorescence indicated that the frontal region of strong deformation contained many vinculin plaques but no apparent concentration of actin or myosin II filaments. Strong mechanical forces in the anterior region, generated by locally activated myosin II and transmitted through vinculin-rich structures, likely play a major role in cell locomotion and in mechanical signaling with the surrounding environment.
Figures
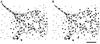


Similar articles
-
Cell locomotion and focal adhesions are regulated by substrate flexibility.Proc Natl Acad Sci U S A. 1997 Dec 9;94(25):13661-5. doi: 10.1073/pnas.94.25.13661. Proc Natl Acad Sci U S A. 1997. PMID: 9391082 Free PMC article.
-
Subcellular tension fields and mechanical resistance of the lamella front related to the direction of locomotion.Cell Biochem Biophys. 1998;29(3):243-62. doi: 10.1007/BF02737897. Cell Biochem Biophys. 1998. PMID: 9868581
-
Molecular dissection of the fibroblast-traction machinery.Cell Motil Cytoskeleton. 2004 Jul;58(3):175-85. doi: 10.1002/cm.20004. Cell Motil Cytoskeleton. 2004. PMID: 15146536
-
Using molecular genetics as a tool in understanding crawling cell locomotion in myoblasts.Biochem Soc Symp. 1999;65:281-99. Biochem Soc Symp. 1999. PMID: 10320945 Review.
-
A new method for application of force to cells via ferric oxide beads.Pflugers Arch. 1998 Jan;435(2):320-7. doi: 10.1007/s004240050518. Pflugers Arch. 1998. PMID: 9382948 Review.
Cited by
-
The tension mounts: stress fibers as force-generating mechanotransducers.J Cell Biol. 2013 Jan 7;200(1):9-19. doi: 10.1083/jcb.201210090. J Cell Biol. 2013. PMID: 23295347 Free PMC article. Review.
-
Techniques for assessing 3-D cell-matrix mechanical interactions in vitro and in vivo.Exp Cell Res. 2013 Oct 1;319(16):2470-80. doi: 10.1016/j.yexcr.2013.06.018. Epub 2013 Jun 29. Exp Cell Res. 2013. PMID: 23819988 Free PMC article. Review.
-
Spatiotemporal organization, regulation, and functions of tractions during neutrophil chemotaxis.Blood. 2010 Oct 28;116(17):3297-310. doi: 10.1182/blood-2009-12-260851. Epub 2010 Jul 8. Blood. 2010. PMID: 20616216 Free PMC article.
-
Decomposition cross-correlation for analysis of collagen matrix deformation by single smooth muscle cells.Med Biol Eng Comput. 2008 May;46(5):443-50. doi: 10.1007/s11517-008-0325-z. Med Biol Eng Comput. 2008. PMID: 18324432 Free PMC article.
-
Multidimensional traction force microscopy reveals out-of-plane rotational moments about focal adhesions.Proc Natl Acad Sci U S A. 2013 Jan 15;110(3):881-6. doi: 10.1073/pnas.1207997110. Epub 2012 Dec 31. Proc Natl Acad Sci U S A. 2013. PMID: 23277584 Free PMC article.
References
-
- Bershadsky A, Chausovsky A, Becker E, Lyubimova A, Geiger B. Involvement of microtubules in the control of adhesion-dependent signal transduction. Curr Biol. 1996;6:1279–1289. - PubMed
-
- Burton K, Taylor DL. Traction forces of cytokinesis measured with optically modified elastic substrata. Nature. 1997;385:450–454. - PubMed
-
- Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276:1425–1428. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

