Uncoupling of transfer of the presequence and unfolding of the mature domain in precursor translocation across the mitochondrial outer membrane
- PMID: 10097089
- PMCID: PMC22346
- DOI: 10.1073/pnas.96.7.3634
Uncoupling of transfer of the presequence and unfolding of the mature domain in precursor translocation across the mitochondrial outer membrane
Abstract
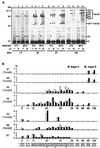
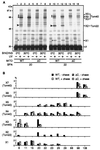
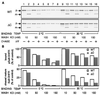
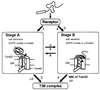
Translocation of mitochondrial precursor proteins across the mitochondrial outer membrane is facilitated by the translocase of the outer membrane (TOM) complex. By using site-specific photocrosslinking, we have mapped interactions between TOM proteins and a mitochondrial precursor protein arrested at two distinct stages, stage A (accumulated at 0 degrees C) and stage B (accumulated at 30 degrees C), in the translocation across the outer membrane at high resolution not achieved previously. Although the stage A and stage B intermediates were assigned previously to the forms bound to the cis site and the trans site of the TOM complex, respectively, the results of crosslinking indicate that the presequence of the intermediates at both stage A and stage B is already on the trans side of the outer membrane. The mature domain is unfolded and bound to Tom40 at stage B whereas it remains folded at stage A. After dissociation from the TOM complex, translocation of the stage B intermediate, but not of the stage A intermediate, across the inner membrane was promoted by the intermembrane-space domain of Tom22. We propose a new model for protein translocation across the outer membrane, where translocation of the presequence and unfolding of the mature domain are not necessarily coupled.
Figures

Similar articles
-
cis and trans sites of the TOM complex of mitochondria in unfolding and initial translocation of preproteins.J Biol Chem. 1998 Apr 10;273(15):8806-13. doi: 10.1074/jbc.273.15.8806. J Biol Chem. 1998. PMID: 9535859
-
Apocytochrome c requires the TOM complex for translocation across the mitochondrial outer membrane.EMBO J. 2001 Oct 15;20(20):5626-35. doi: 10.1093/emboj/20.20.5626. EMBO J. 2001. PMID: 11598006 Free PMC article.
-
Mitochondrial protein import. Tom40 plays a major role in targeting and translocation of preproteins by forming a specific binding site for the presequence.J Biol Chem. 1997 Jul 25;272(30):18725-31. doi: 10.1074/jbc.272.30.18725. J Biol Chem. 1997. PMID: 9228044
-
Protein translocation pathways of the mitochondrion.FEBS Lett. 2000 Jun 30;476(1-2):27-31. doi: 10.1016/s0014-5793(00)01664-1. FEBS Lett. 2000. PMID: 10878244 Review.
-
Unlocking the presequence import pathway.Trends Cell Biol. 2015 May;25(5):265-75. doi: 10.1016/j.tcb.2014.12.001. Epub 2014 Dec 23. Trends Cell Biol. 2015. PMID: 25542066 Review.
Cited by
-
Recognition of preproteins by the isolated TOM complex of mitochondria.EMBO J. 2000 Sep 15;19(18):4895-902. doi: 10.1093/emboj/19.18.4895. EMBO J. 2000. PMID: 10990453 Free PMC article.
-
Coordinated Translocation of Presequence-Containing Precursor Proteins Across Two Mitochondrial Membranes: Knowns and Unknowns of How TOM and TIM23 Complexes Cooperate With Each Other.Front Physiol. 2022 Jan 6;12:806426. doi: 10.3389/fphys.2021.806426. eCollection 2021. Front Physiol. 2022. PMID: 35069261 Free PMC article. Review.
-
Tim50's presequence receptor domain is essential for signal driven transport across the TIM23 complex.J Cell Biol. 2011 Nov 14;195(4):643-56. doi: 10.1083/jcb.201105098. Epub 2011 Nov 7. J Cell Biol. 2011. PMID: 22065641 Free PMC article.
-
Protein import in mitochondria biogenesis: guided by targeting signals and sustained by dedicated chaperones.RSC Adv. 2021 Oct 1;11(51):32476-32493. doi: 10.1039/d1ra04497d. eCollection 2021 Sep 27. RSC Adv. 2021. PMID: 35495482 Free PMC article. Review.
-
An Outer Mitochondrial Translocase, Tom22, Is Crucial for Inner Mitochondrial Steroidogenic Regulation in Adrenal and Gonadal Tissues.Mol Cell Biol. 2016 Jan 19;36(6):1032-47. doi: 10.1128/MCB.01107-15. Mol Cell Biol. 2016. PMID: 26787839 Free PMC article.
References
-
- Omura T. J Biochem. 1998;123:1010–1016. - PubMed
-
- Schatz G. J Biol Chem. 1996;271:31763–31766. - PubMed
-
- Neupert W. Annu Rev Biochem. 1997;66:863–917. - PubMed
-
- Pfanner N, Craig E A, Hölinger A. Annu Rev Cell Dev Biol. 1997;13:25–51. - PubMed
-
- Endo T. In: Molecular Chaperones in the Life Cycle of Proteins. Fink A L, Goto Y, editors. New York: Dekker; 1997. pp. 435–466.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

