Severe impairment in early host defense against Candida albicans in mice deficient in myeloperoxidase
- PMID: 10085024
- PMCID: PMC96534
- DOI: 10.1128/IAI.67.4.1828-1836.1999
Severe impairment in early host defense against Candida albicans in mice deficient in myeloperoxidase
Abstract
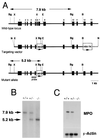
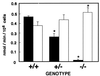
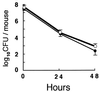
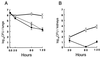
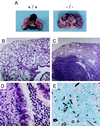
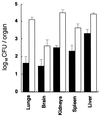
Myeloperoxidase (MPO) catalyzes the reaction of hydrogen peroxide with chloride ion to produce hypochlorous acid (HOCl), which is used for microbial killing by phagocytic cells. Despite the important role of MPO in host defense, however, MPO deficiency is relatively common in humans, and most of these individuals are in good health. To define the in vivo role of MPO, we have generated by gene targeting mice having no MPO activity in their neutrophils and monocytes. The mice without MPO developed normally, were fertile, and showed normal clearance of intraperitoneal Staphylococcus aureus. However, they showed increased susceptibility to pneumonia and death following intratracheal infection with Candida albicans. Furthermore, the lack of MPO significantly enhanced the dissemination of intraperitoneally injected C. albicans into various organs during the first 7 days. Thus, MPO is important for early host defense against fungal infection, and the inability to generate HOCl cannot be compensated for by other oxygen-dependent systems in vivo in mice. The mutant mice serve as a model for studying pulmonary and systemic candidiasis.
Figures

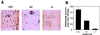
Similar articles
-
Relative contributions of myeloperoxidase and NADPH-oxidase to the early host defense against pulmonary infections with Candida albicans and Aspergillus fumigatus.Med Mycol. 2002 Dec;40(6):557-63. doi: 10.1080/mmy.40.6.557.563. Med Mycol. 2002. PMID: 12521119
-
In vivo role of myeloperoxidase for the host defense.Jpn J Infect Dis. 2004 Oct;57(5):S15. Jpn J Infect Dis. 2004. PMID: 15507755
-
[Role of myeloperoxidase in the host defense against fungal infection].Nihon Ishinkin Gakkai Zasshi. 2006;47(3):195-9. doi: 10.3314/jjmm.47.195. Nihon Ishinkin Gakkai Zasshi. 2006. PMID: 16940954 Review. Japanese.
-
Differential host susceptibility to pulmonary infections with bacteria and fungi in mice deficient in myeloperoxidase.J Infect Dis. 2000 Oct;182(4):1276-9. doi: 10.1086/315843. Epub 2000 Sep 6. J Infect Dis. 2000. PMID: 10979934
-
[Role of neutrophil-derived reactive oxygen species in host defense and inflammation].Med Mycol J. 2012;53(2):123-8. doi: 10.3314/mmj.53.123. Med Mycol J. 2012. PMID: 22728595 Review. Japanese.
Cited by
-
Oxazine conjugated nanoparticle detects in vivo hypochlorous acid and peroxynitrite generation.J Am Chem Soc. 2009 Nov 4;131(43):15739-44. doi: 10.1021/ja903922u. J Am Chem Soc. 2009. PMID: 19817443 Free PMC article.
-
Role of alveolar macrophages in Candida-induced acute lung injury.Clin Diagn Lab Immunol. 2001 Nov;8(6):1258-62. doi: 10.1128/CDLI.8.6.1258-1262.2001. Clin Diagn Lab Immunol. 2001. PMID: 11687472 Free PMC article.
-
The function of the NADPH oxidase of phagocytes and its relationship to other NOXs in plants, invertebrates, and mammals.Int J Biochem Cell Biol. 2008;40(4):604-18. doi: 10.1016/j.biocel.2007.10.003. Epub 2007 Oct 9. Int J Biochem Cell Biol. 2008. PMID: 18036868 Free PMC article. Review.
-
Antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice.J Clin Invest. 2002 Oct;110(7):955-63. doi: 10.1172/JCI15918. J Clin Invest. 2002. PMID: 12370273 Free PMC article.
-
The role of neutrophils in the induction of glomerulonephritis by anti-myeloperoxidase antibodies.Am J Pathol. 2005 Jul;167(1):39-45. doi: 10.1016/S0002-9440(10)62951-3. Am J Pathol. 2005. PMID: 15972950 Free PMC article.
References
-
- Andrews P C, Krinsky N I. The reductive cleavage of myeloperoxidase in half, producing enzymically active hemi-myeloperoxidase. J Biol Chem. 1981;256:4211–4218. - PubMed
-
- Badwey J A, Karnovsky M L. Active oxygen species and the functions of phagocytic leukocytes. Annu Rev Biochem. 1980;49:695–726. - PubMed
-
- Belaaouaj A, McCarthy R, Baumann M, Gao Z, Ley T J, Abraham S N, Shapiro S D. Mice lacking neutrophil elastase reveal impaired host defense against gram negative bacterial sepsis. Nat Med. 1998;4:615–618. - PubMed
-
- Bradley P P, Christensen R D, Rothstein G. Cellular and extracellular myeloperoxidase in pyogenic inflammation. Blood. 1982;60:618–622. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

