The herpes simplex virus type 1 regulatory protein ICP27 is required for the prevention of apoptosis in infected human cells
- PMID: 10074128
- PMCID: PMC104038
- DOI: 10.1128/JVI.73.4.2803-2813.1999
The herpes simplex virus type 1 regulatory protein ICP27 is required for the prevention of apoptosis in infected human cells
Abstract
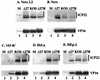
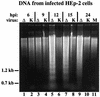
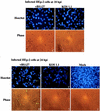
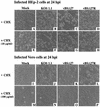
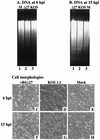
The herpes simplex virus type 1 (HSV-1) ICP27 protein is an immediate-early or alpha protein which is essential for the optimal expression of late genes as well as the synthesis of viral DNA in cultures of Vero cells. Our specific goal was to characterize the replication of a virus incapable of synthesizing ICP27 in cultured human cells. We found that infection with an HSV-1 ICP27 deletion virus of at least three separate strains of human cells did not produce immediate-early or late proteins at the levels observed following wild-type virus infections. Cell morphology, chromatin condensation, and genomic DNA fragmentation measurements demonstrated that the human cells died by apoptosis after infection with the ICP27 deletion virus. These features of the apoptosis were identical to those which occur during wild-type infections of human cells when total protein synthesis has been inhibited. Vero cells infected with the ICP27 deletion virus did not exhibit any of the features of apoptosis. Based on these results, we conclude that while HSV-1 infection likely induced apoptosis in all cells, viral evasion of the response differed among the cells tested in this study.
Figures



Similar articles
-
Induction and prevention of apoptosis in human HEp-2 cells by herpes simplex virus type 1.J Virol. 1999 Dec;73(12):10359-70. doi: 10.1128/JVI.73.12.10359-10370.1999. J Virol. 1999. PMID: 10559354 Free PMC article.
-
Accumulation of herpes simplex virus type 1 early and leaky-late proteins correlates with apoptosis prevention in infected human HEp-2 cells.J Virol. 2001 Jan;75(2):1013-30. doi: 10.1128/JVI.75.2.1013-1030.2001. J Virol. 2001. PMID: 11134315 Free PMC article.
-
Stress Induced Transcription Factors Transactivate the Herpes Simplex Virus 1 Infected Cell Protein 27 (ICP27) Transcriptional Enhancer.Viruses. 2021 Nov 17;13(11):2296. doi: 10.3390/v13112296. Viruses. 2021. PMID: 34835102 Free PMC article.
-
Aberrant RNA polymerase initiation and processivity on the genome of a herpes simplex virus 1 mutant lacking ICP27.J Virol. 2024 Jun 13;98(6):e0071224. doi: 10.1128/jvi.00712-24. Epub 2024 May 23. J Virol. 2024. PMID: 38780246 Free PMC article.
-
Control of VP16 translation by the herpes simplex virus type 1 immediate-early protein ICP27.J Virol. 2005 Apr;79(7):4120-31. doi: 10.1128/JVI.79.7.4120-4131.2005. J Virol. 2005. PMID: 15767413 Free PMC article.
Cited by
-
The Race between Host Antiviral Innate Immunity and the Immune Evasion Strategies of Herpes Simplex Virus 1.Microbiol Mol Biol Rev. 2020 Sep 30;84(4):e00099-20. doi: 10.1128/MMBR.00099-20. Print 2020 Nov 18. Microbiol Mol Biol Rev. 2020. PMID: 32998978 Free PMC article. Review.
-
Infection of cattle with a bovine herpesvirus 1 strain that contains a mutation in the latency-related gene leads to increased apoptosis in trigeminal ganglia during the transition from acute infection to latency.J Virol. 2003 Apr;77(8):4848-57. doi: 10.1128/jvi.77.8.4848-4857.2003. J Virol. 2003. PMID: 12663791 Free PMC article.
-
Programmed Cell Death-Dependent Host Defense in Ocular Herpes Simplex Virus Infection.Front Microbiol. 2022 Apr 8;13:869064. doi: 10.3389/fmicb.2022.869064. eCollection 2022. Front Microbiol. 2022. PMID: 35464953 Free PMC article. Review.
-
Oligonucleotides designed to inhibit TLR9 block Herpes simplex virus type 1 infection at multiple steps.Antiviral Res. 2014 Sep;109:83-96. doi: 10.1016/j.antiviral.2014.06.015. Epub 2014 Jul 1. Antiviral Res. 2014. PMID: 24995383 Free PMC article.
-
Virus-mediated inactivation of anti-apoptotic Bcl-2 family members promotes Gasdermin-E-dependent pyroptosis in barrier epithelial cells.Immunity. 2021 Jul 13;54(7):1447-1462.e5. doi: 10.1016/j.immuni.2021.04.012. Epub 2021 May 11. Immunity. 2021. PMID: 33979579 Free PMC article.
References
-
- Aubert, M., and J. A. Blaho. Unpublished data.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

