An antiviral mechanism of nitric oxide: inhibition of a viral protease
- PMID: 10023767
- PMCID: PMC7129050
- DOI: 10.1016/s1074-7613(00)80003-5
An antiviral mechanism of nitric oxide: inhibition of a viral protease
Abstract
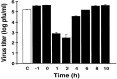
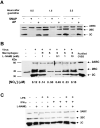
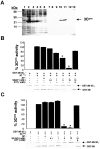
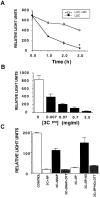
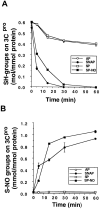
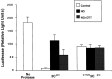
Although nitric oxide (NO) kills or inhibits the replication of a variety of intracellular pathogens, the antimicrobial mechanisms of NO are unknown. Here, we identify a viral protease as a target of NO. The life cycle of many viruses depends upon viral proteases that cleave viral polyproteins into individual polypeptides. NO inactivates the Coxsackievirus protease 3C, an enzyme necessary for the replication of Coxsackievirus. NO S-nitrosylates the cysteine residue in the active site of protease 3C, inhibiting protease activity and interrupting the viral life cycle. Substituting a serine residue for the active site cysteine renders protease 3C resistant to NO inhibition. Since cysteine proteases are critical for virulence or replication of many viruses, bacteria, and parasites, S-nitrosylation of pathogen cysteine proteases may be a general mechanism of antimicrobial host defenses.
Figures
Similar articles
-
Synthesis and evaluation of pyrazolone compounds as SARS-coronavirus 3C-like protease inhibitors.Bioorg Med Chem. 2010 Nov 15;18(22):7849-54. doi: 10.1016/j.bmc.2010.09.050. Epub 2010 Sep 25. Bioorg Med Chem. 2010. PMID: 20947359 Free PMC article.
-
Nitric oxide inhibits dystrophin proteolysis by coxsackieviral protease 2A through S-nitrosylation: A protective mechanism against enteroviral cardiomyopathy.Circulation. 2000 Oct 31;102(18):2276-81. doi: 10.1161/01.cir.102.18.2276. Circulation. 2000. PMID: 11056105
-
N-Terminomics TAILS Identifies Host Cell Substrates of Poliovirus and Coxsackievirus B3 3C Proteinases That Modulate Virus Infection.J Virol. 2018 Mar 28;92(8):e02211-17. doi: 10.1128/JVI.02211-17. Print 2018 Apr 15. J Virol. 2018. PMID: 29437971 Free PMC article.
-
Human rhinovirus 3C protease as a potential target for the development of antiviral agents.Curr Protein Pept Sci. 2007 Feb;8(1):19-27. doi: 10.2174/138920307779941523. Curr Protein Pept Sci. 2007. PMID: 17305557 Review.
-
The SARS-coronavirus papain-like protease: structure, function and inhibition by designed antiviral compounds.Antiviral Res. 2015 Mar;115:21-38. doi: 10.1016/j.antiviral.2014.12.015. Epub 2014 Dec 29. Antiviral Res. 2015. PMID: 25554382 Free PMC article. Review.
Cited by
-
Human and Mouse Eosinophils Have Antiviral Activity against Parainfluenza Virus.Am J Respir Cell Mol Biol. 2016 Sep;55(3):387-94. doi: 10.1165/rcmb.2015-0405OC. Am J Respir Cell Mol Biol. 2016. PMID: 27049514 Free PMC article.
-
Aging and Interferons: Impacts on Inflammation and Viral Disease Outcomes.Cells. 2021 Mar 23;10(3):708. doi: 10.3390/cells10030708. Cells. 2021. PMID: 33806810 Free PMC article. Review.
-
Suppressed anti-inflammatory heat shock response in high-risk COVID-19 patients: lessons from basic research (inclusive bats), light on conceivable therapies.Clin Sci (Lond). 2020 Aug 14;134(15):1991-2017. doi: 10.1042/CS20200596. Clin Sci (Lond). 2020. PMID: 32749472 Free PMC article. Review.
-
Src family kinases participate in the regulation of encephalomyocarditis virus-induced cyclooxygenase-2 expression by macrophages.J Gen Virol. 2010 Sep;91(Pt 9):2278-85. doi: 10.1099/vir.0.022665-0. Epub 2010 May 26. J Gen Virol. 2010. PMID: 20505008 Free PMC article.
-
Nitric oxide in dengue and dengue haemorrhagic fever: necessity or nuisance?FEMS Immunol Med Microbiol. 2009 Jun;56(1):9-24. doi: 10.1111/j.1574-695X.2009.00544.x. Epub 2009 Feb 23. FEMS Immunol Med Microbiol. 2009. PMID: 19239490 Free PMC article. Review.
References
-
- Aldape K, Huizinga H, Bouvier J, McKerrow J. Naegleria fowleri: characterization of a secreted histolytic cysteine protease. Exper. Parasitol. 1994;78:230–241. - PubMed
-
- Babe L.M, Craik C.S. Viral proteases: evolution of diverse structural motifs to optimize function. Cell. 1997;91:427–430. - PubMed
-
- Bailly E, Jambou R, Savel J, Jaureguiberry G. Plasmodium falciparum: differential sensitivity in vitro to E-64 (cysteine protease inhibitor) and Pepstatin A (aspartyl protease inhibitor) J. Protozool. 1992;39:593–599. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

