Immunogenicity and in vitro protective efficacy of a recombinant multistage Plasmodium falciparum candidate vaccine
- PMID: 9990073
- PMCID: PMC15536
- DOI: 10.1073/pnas.96.4.1615
Immunogenicity and in vitro protective efficacy of a recombinant multistage Plasmodium falciparum candidate vaccine
Abstract
Compared with a single-stage antigen-based vaccine, a multistage and multivalent Plasmodium falciparum vaccine would be more efficacious by inducing "multiple layers" of immunity. We have constructed a synthetic gene that encodes for 12 B cell, 6 T cell proliferative, and 3 cytotoxic T lymphocyte epitopes derived from 9 stage-specific P. falciparum antigens corresponding to the sporozoite, liver, erythrocytic asexual, and sexual stages. The gene was expressed in the baculovirus system, and a 41-kDa antigen, termed CDC/NIIMALVAC-1, was purified. Immunization in rabbits with the purified protein in the presence of different adjuvants generated antibody responses that recognized vaccine antigen, linear peptides contained in the vaccine, and all stages of P. falciparum. In vitro assays of protection revealed that the vaccine-elicited antibodies strongly inhibited sporozoite invasion of hepatoma cells and growth of blood-stage parasites in the presence of monocytes. These observations demonstrate that a multicomponent, multistage malaria vaccine can induce immune responses that inhibit parasite development at multiple stages. The rationale and approach used in the development of a multicomponent P. falciparum vaccine will be useful in the development of a multispecies human malaria vaccine and vaccines against other infectious diseases.
Figures
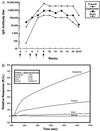
Comment in
-
Malaria vaccines.Proc Natl Acad Sci U S A. 1999 Feb 16;96(4):1167-9. doi: 10.1073/pnas.96.4.1167. Proc Natl Acad Sci U S A. 1999. PMID: 9989994 Free PMC article. Review. No abstract available.
Similar articles
-
Immunogenicity and in vitro protective efficacy of a polyepitope Plasmodium falciparum candidate vaccine constructed by epitope shuffling.Vaccine. 2007 Jul 9;25(28):5155-65. doi: 10.1016/j.vaccine.2007.04.085. Epub 2007 May 21. Vaccine. 2007. PMID: 17548134
-
Irradiated sporozoite vaccine induces HLA-B8-restricted cytotoxic T lymphocyte responses against two overlapping epitopes of the Plasmodium falciparum sporozoite surface protein 2.J Exp Med. 1995 Nov 1;182(5):1435-45. doi: 10.1084/jem.182.5.1435. J Exp Med. 1995. PMID: 7595214 Free PMC article.
-
Immunogenicity of a recombinant malaria vaccine candidate, domain I+II of AMA-1 ectodomain, from Indian P. falciparum alleles.Vaccine. 2008 Aug 18;26(35):4526-35. doi: 10.1016/j.vaccine.2008.06.031. Epub 2008 Jun 30. Vaccine. 2008. PMID: 18590786
-
Current Challenges in the Identification of Pre-Erythrocytic Malaria Vaccine Candidate Antigens.Front Immunol. 2020 Feb 21;11:190. doi: 10.3389/fimmu.2020.00190. eCollection 2020. Front Immunol. 2020. PMID: 32153565 Free PMC article. Review.
-
T cell responses to repeat and non-repeat regions of the circumsporozoite protein detected in volunteers immunized with Plasmodium falciparum sporozoites.Mem Inst Oswaldo Cruz. 1992;87 Suppl 3:223-7. doi: 10.1590/s0074-02761992000700037. Mem Inst Oswaldo Cruz. 1992. PMID: 1364202 Review.
Cited by
-
Malaria vaccines.Proc Natl Acad Sci U S A. 1999 Feb 16;96(4):1167-9. doi: 10.1073/pnas.96.4.1167. Proc Natl Acad Sci U S A. 1999. PMID: 9989994 Free PMC article. Review. No abstract available.
-
Elucidation of immunological response and its regulatory network by P-TUFT-ALT-2: a promising fusion protein vaccine for human lymphatic filariasis.R Soc Open Sci. 2018 May 16;5(5):172039. doi: 10.1098/rsos.172039. eCollection 2018 May. R Soc Open Sci. 2018. PMID: 29892388 Free PMC article.
-
Onchocerca volvulus glycolytic enzyme fructose-1,6-bisphosphate aldolase as a target for a protective immune response in humans.Infect Immun. 2002 Feb;70(2):851-8. doi: 10.1128/IAI.70.2.851-858.2002. Infect Immun. 2002. PMID: 11796620 Free PMC article.
-
Immunization with a combination of merozoite surface proteins 4/5 and 1 enhances protection against lethal challenge with Plasmodium yoelii.Infect Immun. 2002 Dec;70(12):6606-13. doi: 10.1128/IAI.70.12.6606-6613.2002. Infect Immun. 2002. PMID: 12438332 Free PMC article.
-
A region of Plasmodium falciparum antigen Pfs25 that is the target of highly potent transmission-blocking antibodies.Infect Immun. 2000 Oct;68(10):5530-8. doi: 10.1128/IAI.68.10.5530-5538.2000. Infect Immun. 2000. PMID: 10992450 Free PMC article.
References
-
- World Health Organization. Weekly Epidemiol Res. 1989;32:241–247.
-
- Institute of Medicine. Malaria: Obstacles and Opportunities. Washington, DC: Natl. Acad. Press; 1989. pp. 1–6.
-
- Hoffman S L. Malaria Vaccine Development: A Multi-immune Response Approach. Washington, DC: Am. Soc. Microbiol.; 1996. pp. 1–12.
-
- Patarroyo M E, Amador R, Calvijo P, Moreno A, Guzman F, Romero P, Tascon F A, Murillo L A, Ponton G, Trujillo G. Nature (London) 1988;332:158–161. - PubMed
-
- D’Alessandro U, Leach A, Drakeley C J, Bennett S, Olaleye B O, Fegan G W, Jawara M, Langerock P, George M O, Targe G A. Lancet. 1995;346:462–467. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

