Moloney murine leukemia virus infects cells of the developing hair follicle after neonatal subcutaneous inoculation in mice
- PMID: 9971836
- PMCID: PMC104498
- DOI: 10.1128/JVI.73.3.2509-2516.1999
Moloney murine leukemia virus infects cells of the developing hair follicle after neonatal subcutaneous inoculation in mice
Abstract
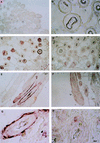
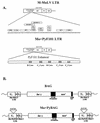
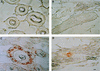
The nature of Moloney murine leukemia virus (M-MuLV) infection after a subcutaneous (s.c.) inoculation was studied. We have previously shown that an enhancer variant of M-MuLV, Mo+PyF101 M-MuLV, is poorly leukemogenic when used to inoculate mice s.c., but not when inoculated intraperitoneally. This attenuation of leukemogenesis correlated with an inability of Mo+PyF101 M-MuLV to establish infection in the bone marrow of mice at early times postinfection. These results suggested that a cell type(s) is infected in the skin by wild-type but not Mo+PyF101 M-MuLV after s.c. inoculation and that this infection is important for the delivery of infection to the bone marrow, as well as for efficient leukemogenesis. To determine the nature of the cell types infected by M-MuLV and Mo+PyF101 M-MuLV in the skin after a s.c. inoculation, immunohistochemistry with an anti-M-MuLV CA antibody was performed. Cells of developing hair follicles, specifically cells of the outer root sheath (ORS), were extensively infected by M-MuLV after s.c. inoculation. The Mo+PyF101 M-MuLV variant also infected cells of the ORS but the level of infection was lower. By Western blot analysis, the level of infection in skin by Mo+PyF101 M-MuLV was approximately 4- to 10-fold less than that of wild-type M-MuLV. Similar results were seen when a mouse keratinocyte line was infected in vitro with both viruses. Cells of the ORS are a primary target of infection in vivo, since a replication defective M-MuLV-based vector expressing beta-galactosidase also infected these cells after a s.c. inoculation.
Figures


Similar articles
-
Differential behavior of the Mo + PyF101 enhancer variant of Moloney murine leukemia virus in rats and mice.Virology. 1998 Mar 1;242(1):60-7. doi: 10.1006/viro.1997.9007. Virology. 1998. PMID: 9501051
-
Recombinant mink cell focus-inducing virus and long terminal repeat alterations accompany the increased leukemogenicity of the Mo+PyF101 variant of Moloney murine leukemia virus after intraperitoneal inoculation.J Virol. 1995 Feb;69(2):1037-43. doi: 10.1128/JVI.69.2.1037-1043.1995. J Virol. 1995. PMID: 7815481 Free PMC article.
-
Appearance of mink cell focus-inducing recombinants during in vivo infection by moloney murine leukemia virus (M-MuLV) or the Mo+PyF101 M-MuLV enhancer variant: implications for sites of generation and roles in leukemogenesis.J Virol. 1999 Jul;73(7):5671-80. doi: 10.1128/JVI.73.7.5671-5680.1999. J Virol. 1999. PMID: 10364317 Free PMC article.
-
The leukemogenic potential of an enhancer variant of Moloney murine leukemia virus varies with the route of inoculation.J Virol. 1994 Nov;68(11):6883-9. doi: 10.1128/JVI.68.11.6883-6889.1994. J Virol. 1994. PMID: 7933068 Free PMC article.
-
Effects of nonleukemogenic and wild-type Moloney murine leukemia virus on lymphoid cells in vivo: identification of a preleukemic shift in thymocyte subpopulations.J Virol. 1986 Nov;60(2):423-30. doi: 10.1128/JVI.60.2.423-430.1986. J Virol. 1986. PMID: 3490580 Free PMC article.
Cited by
-
Wavy changes in the whiskers of domestic cats are correlated with feline leukemia virus infection.BMC Vet Res. 2023 Mar 4;19(1):58. doi: 10.1186/s12917-023-03610-7. BMC Vet Res. 2023. PMID: 36871053 Free PMC article.
References
-
- Brightman B K, Okimoto M, Kulkarni V, Lander J K, Fan H. Differential behavior of the Mo+PyF101 enhancer variant of Moloney murine leukemia virus in rats and mice. Virology. 1998;242:60–67. - PubMed
-
- Chesebro B, Britt W, Evans L, Wehrly K, Nishio J, Cloyd M. Characterization of monoclonal antibodies reactive with murine leukemia viruses: use in analysis of strains of friend MCF and Friend ecotropic murine leukemia virus. Virology. 1983;127:134–148. - PubMed
-
- Coffin J, Hughes S H, Varmus H, editors. Retroviruses. Plainview, N.Y: Cold Spring Harbor Press; 1997. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

