Hepatitis C virus core protein enhances NF-kappaB signal pathway triggering by lymphotoxin-beta receptor ligand and tumor necrosis factor alpha
- PMID: 9882379
- PMCID: PMC103998
- DOI: 10.1128/JVI.73.2.1672-1681.1999
Hepatitis C virus core protein enhances NF-kappaB signal pathway triggering by lymphotoxin-beta receptor ligand and tumor necrosis factor alpha
Abstract
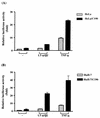
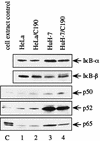
Our previous study indicated that the core protein of hepatitis C virus (HCV) can associate with tumor necrosis factor receptor (TNFR)-related lymphotoxin-beta receptor (LT-betaR) and that this protein-protein interaction plays a modulatory effect on the cytolytic activity of recombinant form LT-betaR ligand (LT-alpha1beta2) but not tumor necrosis factor alpha (TNF-alpha) in certain cell types. Since both TNF-alpha/TNFR and LT-alpha1beta2/LT-betaR are also engaged in transcriptional activator NF-kappaB activation or c-Jun N-terminal kinase (JNK) activation, the biological effects of the HCV core protein on these regards were elucidated in this study. As demonstrated by the electrophoretic mobility shift assay, the expression of HCV core protein prolonged or enhanced the TNF-alpha or LT-alpha1beta2-induced NF-kappaB DNA-binding activity in HuH-7 and HeLa cells. The presence of HCV core protein in HeLa or HuH-7 cells with or without cytokine treatment also enhanced the NF-kappaB-dependent reporter plasmid activity, and this effect was more strongly seen with HuH-7 cells than with HeLa cells. Western blot analysis suggested that this modulation of the NF-kappaB activity by the HCV core protein was in part due to elevated or prolonged nuclear retention of p50 or p65 species of NF-kappaB in core protein-producing cells with or without cytokine treatment. Furthermore, the HCV core protein enhanced or prolonged the IkappaB-beta degradation triggering by TNF-alpha or LT-alpha1beta2 both in HeLa and HuH-7 cells. In contrast to that of IkappaB-beta, the increased degradation of IkappaB-alpha occurred only in LT-alpha1beta2-treated core-producing HeLa cells and not in TNF-alpha-treated cells. Therefore, the HCV core protein plays a modulatory effect on NF-kappaB activation triggering by both cytokines, though the mechanism of NF-kappaB activation, in particular the regulation of IkappaB degradation, is rather cell line and cytokine specific. Studies also suggested that the HCV core protein had no effect on TNF-alpha-stimulated JNK activity in both HeLa and HuH-7 cells. These findings, together with our previous study, strongly suggest that among three signaling pathways triggered by the TNF-alpha-related cytokines, the HCV core protein potentiates NF-kappaB activation in most cell types, which in turn may contribute to the chronically activated, persistent state of HCV-infected cells.
Figures
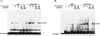
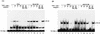


Similar articles
-
Direct interaction of hepatitis C virus core protein with the cellular lymphotoxin-beta receptor modulates the signal pathway of the lymphotoxin-beta receptor.J Virol. 1997 Dec;71(12):9417-26. doi: 10.1128/JVI.71.12.9417-9426.1997. J Virol. 1997. PMID: 9371602 Free PMC article.
-
Signaling through the lymphotoxin-beta receptor stimulates HIV-1 replication alone and in cooperation with soluble or membrane-bound TNF-alpha.J Immunol. 1999 May 15;162(10):6016-23. J Immunol. 1999. PMID: 10229841
-
Ectopic expression of hepatitis C virus core protein differentially regulates nuclear transcription factors.J Virol. 1998 Dec;72(12):9722-8. doi: 10.1128/JVI.72.12.9722-9728.1998. J Virol. 1998. PMID: 9811706 Free PMC article.
-
Mechanisms of action of the tumor necrosis factor and lymphotoxin ligand-receptor system.Eur Cytokine Netw. 1995 Mar-Apr;6(2):83-96. Eur Cytokine Netw. 1995. PMID: 7578992 Review.
-
TLR-4, IL-1R and TNF-R signaling to NF-kappaB: variations on a common theme.Cell Mol Life Sci. 2008 Oct;65(19):2964-78. doi: 10.1007/s00018-008-8064-8. Cell Mol Life Sci. 2008. PMID: 18535784 Free PMC article. Review.
Cited by
-
Hepatitis C virus-related hepatocellular carcinoma: An insight into molecular mechanisms and therapeutic strategies.World J Hepatol. 2012 Dec 27;4(12):342-55. doi: 10.4254/wjh.v4.i12.342. World J Hepatol. 2012. PMID: 23355912 Free PMC article.
-
Core protein-coding sequence, but not core protein, modulates the efficiency of cap-independent translation directed by the internal ribosome entry site of hepatitis C virus.J Virol. 2000 Dec;74(23):11347-58. doi: 10.1128/jvi.74.23.11347-11358.2000. J Virol. 2000. PMID: 11070035 Free PMC article.
-
Genetic clustering of hepatitis C virus strains and severity of recurrent hepatitis after liver transplantation.J Virol. 2001 Dec;75(23):11292-7. doi: 10.1128/JVI.75.23.11292-11297.2001. J Virol. 2001. PMID: 11689609 Free PMC article.
-
B-cell-intrinsic hepatitis C virus expression leads to B-cell-lymphomagenesis and induction of NF-κB signalling.PLoS One. 2014 Mar 20;9(3):e91373. doi: 10.1371/journal.pone.0091373. eCollection 2014. PLoS One. 2014. PMID: 24651473 Free PMC article.
-
Toll-like Receptor Response to Hepatitis C Virus Infection: A Recent Overview.Int J Mol Sci. 2022 May 13;23(10):5475. doi: 10.3390/ijms23105475. Int J Mol Sci. 2022. PMID: 35628287 Free PMC article. Review.
References
-
- Aggarwal B B, Natarajan K. Tumor necrosis factors: developments during the last decade. Eur Cytokine Netw. 1996;7:93–124. - PubMed
-
- Arizawa S, Scheffrahn I, Mosialos G, Brand H, Duyster J, Kaye K, Harada J, Dougall B, Hubinger G, Kieff E, Herrmann F, Leutz A, Gruss H J. Tumor necrosis factor receptor-associated factor (TRAF) 5 and TRAF2 are involved in CD30-mediated NF-κB activation. J Biol Chem. 1997;272:2042–2045. - PubMed
-
- Baens M, Chaffanet M, Cassiman J J, Den Berghe H, Marynen P. Construction and evaluation of a HNcDNA library of human 12p transcribed sequences derived from a somatic cell hybrid. Genomics. 1993;16:214–218. - PubMed
-
- Baeuerle P A, Baltimore D. NF-κB: ten years after. Cell. 1996;87:13–20. - PubMed
-
- Baeuerle P A, Henkel T. Function and activation of NF-κB in the immune system. Annu Rev Immunol. 1994;12:141–179. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

