Feline calicivirus capsid protein expression and capsid assembly in cultured feline cells
- PMID: 9847398
- PMCID: PMC103899
- DOI: 10.1128/JVI.73.1.834-838.1999
Feline calicivirus capsid protein expression and capsid assembly in cultured feline cells
Abstract
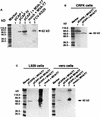
The capsid protein of feline calicivirus (FCV) was expressed by using plasmids containing cytomegalovirus, simian virus 40, or T7 promoters. The strongest expression was achieved with the T7 promoter and coinfection with vaccinia virus expressing the T7 RNA polymerase (MVA/T7pol). The FCV precursor capsid protein was processed to the mature-size protein, and these proteins were assembled in to virus-like particles.
Figures

Similar articles
-
Feline calicivirus capsid protein expression and self-assembly in cultured feline cells.Vet Microbiol. 1999 Sep 1;69(1-2):63-6. doi: 10.1016/s0378-1135(99)00089-9. Vet Microbiol. 1999. PMID: 10515271
-
Feline calicivirus VP2 is involved in the self-assembly of the capsid protein into virus-like particles.Res Vet Sci. 2010 Oct;89(2):279-81. doi: 10.1016/j.rvsc.2010.03.011. Epub 2010 Apr 1. Res Vet Sci. 2010. PMID: 20362313
-
Assembly of feline calicivirus-like particle and its immunogenicity.Vet Microbiol. 2007 Feb 25;120(1-2):173-8. doi: 10.1016/j.vetmic.2006.10.021. Epub 2006 Oct 25. Vet Microbiol. 2007. PMID: 17126499
-
The subgenomic RNA of feline calicivirus is packaged into viral particles during infection.Virus Res. 2002 Jul;87(1):89-93. doi: 10.1016/s0168-1702(02)00086-2. Virus Res. 2002. PMID: 12135793
-
Feline calicivirus.Vet Res. 2007 Mar-Apr;38(2):319-35. doi: 10.1051/vetres:2006056. Epub 2007 Feb 13. Vet Res. 2007. PMID: 17296159 Review.
Cited by
-
Highs and Lows in Calicivirus Reverse Genetics.Viruses. 2024 May 28;16(6):866. doi: 10.3390/v16060866. Viruses. 2024. PMID: 38932159 Free PMC article. Review.
-
Implementation of an Immunoassay Based on the MVA-T7pol-Expression System for Rapid Identification of Immunogenic SARS-CoV-2 Antigens: A Proof-of-Concept Study.Int J Mol Sci. 2024 Oct 10;25(20):10898. doi: 10.3390/ijms252010898. Int J Mol Sci. 2024. PMID: 39456680 Free PMC article.
-
Conserved Surface Residues on the Feline Calicivirus Capsid Are Essential for Interaction with Its Receptor Feline Junctional Adhesion Molecule A (fJAM-A).J Virol. 2018 Mar 28;92(8):e00035-18. doi: 10.1128/JVI.00035-18. Print 2018 Apr 15. J Virol. 2018. PMID: 29386293 Free PMC article.
-
Multiple antigenic sites are involved in blocking the interaction of GII.4 norovirus capsid with ABH histo-blood group antigens.J Virol. 2012 Jul;86(13):7414-26. doi: 10.1128/JVI.06729-11. Epub 2012 Apr 24. J Virol. 2012. PMID: 22532688 Free PMC article.
-
Norwalk virus open reading frame 3 encodes a minor structural protein.J Virol. 2000 Jul;74(14):6581-91. doi: 10.1128/jvi.74.14.6581-6591.2000. J Virol. 2000. PMID: 10864672 Free PMC article.
References
-
- Carroll M W, Moss B. Host range and cytopathogenicity of the highly attenuated MVA strain of vaccinia virus: propagation and generation of recombinant viruses in a non-human mammalian cell line. Virology. 1997;238:198–211. - PubMed
-
- DeSilver D A, Guimond P M, Gibson J K, Thomsen D R, Wardley R C, Lowery D E. Expression of the complete capsid and the hypervariable region of feline calicivirus in the baculovirus expression system. In: Chasey D, Gaskell R M, Clarke I N, editors. Proceedings of the First International Symposium on Caliciviruses. Reading, United Kingdom: European Society for Veterinary Virology; 1997. pp. 131–143.
-
- Drexler I, Heller K, Wahren B, Erfle V, Sutter G. Highly attenuated modified vaccinia virus Ankara replicates in baby hamster kidney cells, a potential host for virus propagation, but not in various human transformed and primary cells. J Gen Virol. 1998;79:347–352. - PubMed
-
- Geissler K, Schneider K, Platzer G, Truyen B, Kaaden O-R, Truyen U. Genetic and antigenic heterogeneity among feline calicivirus isolates from distinct disease manifestations. Virus Res. 1997;48:193–206. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

