Retrograde transport from the pre-Golgi intermediate compartment and the Golgi complex is affected by the vacuolar H+-ATPase inhibitor bafilomycin A1
- PMID: 9843588
- PMCID: PMC25677
- DOI: 10.1091/mbc.9.12.3561
Retrograde transport from the pre-Golgi intermediate compartment and the Golgi complex is affected by the vacuolar H+-ATPase inhibitor bafilomycin A1
Abstract
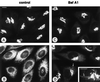
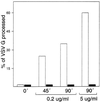
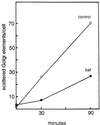
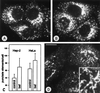
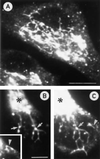
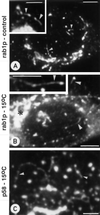
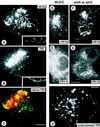
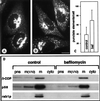
The effect of the vacuolar H+-ATPase inhibitor bafilomycin A1 (Baf A1) on the localization of pre-Golgi intermediate compartment (IC) and Golgi marker proteins was used to study the role of acidification in the function of early secretory compartments. Baf A1 inhibited both brefeldin A- and nocodazole-induced retrograde transport of Golgi proteins to the endoplasmic reticulum (ER), whereas anterograde ER-to-Golgi transport remained largely unaffected. Furthermore, p58/ERGIC-53, which normally cycles between the ER, IC, and cis-Golgi, was arrested in pre-Golgi tubules and vacuoles, and the number of p58-positive approximately 80-nm Golgi (coatomer protein I) vesicles was reduced, suggesting that the drug inhibits the retrieval of the protein from post-ER compartments. In parallel, redistribution of beta-coatomer protein from the Golgi to peripheral pre-Golgi structures took place. The small GTPase rab1p was detected in short pre-Golgi tubules in control cells and was efficiently recruited to the tubules accumulating in the presence of Baf A1. In contrast, these tubules showed no enrichment of newly synthesized, anterogradely transported proteins, indicating that they participate in retrograde transport. These results suggest that the pre-Golgi structures contain an active H+-ATPase that regulates retrograde transport at the ER-Golgi boundary. Interestingly, although Baf A1 had distinct effects on peripheral pre-Golgi structures, only more central, p58-containing elements accumulated detectable amounts of 3-(2, 4-dinitroanilino)-3'-amino-N-methyldipropylamine (DAMP), a marker for acidic compartments, raising the possibility that the lumenal pH of the pre-Golgi structures gradually changes in parallel with their translocation to the Golgi region.
Figures


Similar articles
-
Localization of the small GTP-binding protein rab1p to early compartments of the secretory pathway.J Cell Sci. 1995 Apr;108 ( Pt 4):1541-52. doi: 10.1242/jcs.108.4.1541. J Cell Sci. 1995. PMID: 7615674
-
Active vacuolar H+ATPase is required for both endocytic and exocytic processes during viral infection of BHK-21 cells.J Biol Chem. 1994 Jul 1;269(26):17577-85. J Biol Chem. 1994. PMID: 8021266
-
The p58-positive pre-golgi intermediates consist of distinct subpopulations of particles that show differential binding of COPI and COPII coats and contain vacuolar H(+)-ATPase.J Cell Sci. 2000 Oct;113 ( Pt 20):3623-38. doi: 10.1242/jcs.113.20.3623. J Cell Sci. 2000. PMID: 11017878
-
Membrane cycling between the ER and Golgi apparatus and its role in biosynthetic transport.Subcell Biochem. 1993;21:95-119. doi: 10.1007/978-1-4615-2912-5_5. Subcell Biochem. 1993. PMID: 8256276 Review.
-
Protein sorting by directed maturation of Golgi compartments.Science. 1999 Jul 2;285(5424):63-6. doi: 10.1126/science.285.5424.63. Science. 1999. PMID: 10390362 Review.
Cited by
-
Proteoglycan synthesis and Golgi organization in polarized epithelial cells.J Histochem Cytochem. 2012 Dec;60(12):926-35. doi: 10.1369/0022155412461256. Epub 2012 Sep 1. J Histochem Cytochem. 2012. PMID: 22941419 Free PMC article. Review.
-
The function of the intermediate compartment in pre-Golgi trafficking involves its stable connection with the centrosome.Mol Biol Cell. 2009 Oct;20(20):4458-70. doi: 10.1091/mbc.e08-12-1229. Epub 2009 Aug 26. Mol Biol Cell. 2009. PMID: 19710425 Free PMC article.
-
Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication.Nat Cell Biol. 2019 Jan;21(1):9-17. doi: 10.1038/s41556-018-0250-9. Epub 2019 Jan 2. Nat Cell Biol. 2019. PMID: 30602770 Review.
-
Models of Intracellular Transport: Pros and Cons.Front Cell Dev Biol. 2019 Aug 7;7:146. doi: 10.3389/fcell.2019.00146. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 31440506 Free PMC article.
-
SNAREs: cogs and coordinators in signaling and development.Plant Physiol. 2008 Aug;147(4):1504-15. doi: 10.1104/pp.108.121129. Plant Physiol. 2008. PMID: 18678742 Free PMC article. Review. No abstract available.
References
-
- Anderson RGW, Pathak RK. Vesicles and cisternae in the trans Golgi apparatus of human fibroblasts are acidic compartments. Cell. 1985;40:635–643. - PubMed
-
- Arar C, Carpentier V, Le Caer J-P, Monsigny M, Legrand A, Roche A-A. ERGIC-53, a membrane protein of the endoplasmic reticulum-Golgi intermediate compartment, is identical to MR60, an intracellular mannose-specific lectin of myelomonocytic cells. J Biol Chem. 1995;270:3551–3553. - PubMed
-
- Aridor M, Balch WE. Principles of selective transport: coat complexes hold the key. Trends Cell Biol. 1996;96:315–320. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

