Phosphoinositide signaling pathways in nuclei are associated with nuclear speckles containing pre-mRNA processing factors
- PMID: 9843587
- PMCID: PMC25675
- DOI: 10.1091/mbc.9.12.3547
Phosphoinositide signaling pathways in nuclei are associated with nuclear speckles containing pre-mRNA processing factors
Abstract
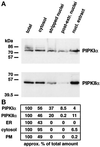
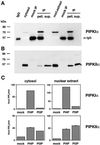
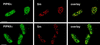
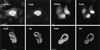
Phosphoinositide signal transduction pathways in nuclei use enzymes that are indistinguishable from their cytosolic analogues. We demonstrate that distinct phosphatidylinositol phosphate kinases (PIPKs), the type I and type II isoforms, are concentrated in nuclei of mammalian cells. The cytosolic and nuclear PIPKs display comparable activities toward the substrates phosphatidylinositol 4-phosphate and phosphatidylinositol 3-phosphate. Indirect immunofluorescence revealed that these kinases were associated with distinct subnuclear domains, identified as "nuclear speckles," which also contained pre-mRNA processing factors. A pool of nuclear phosphatidylinositol bisphosphate (PIP2), the product of these kinases, was also detected at these same sites by monoclonal antibody staining. The localization of PIPKs and PIP2 to speckles is dynamic in that both PIPKs and PIP2 reorganize along with other speckle components upon inhibition of mRNA transcription. Because PIPKs have roles in the production of most phosphatidylinositol second messengers, these findings demonstrate that phosphatidylinositol signaling pathways are localized at nuclear speckles. Surprisingly, the PIPKs and PIP2 are not associated with invaginations of the nuclear envelope or any nuclear membrane structure. The putative absence of membranes at these sites suggests novel mechanisms for the generation of phosphoinositides within these structures.
Figures





Similar articles
-
IQGAP1 is a phosphoinositide effector and kinase scaffold.Adv Biol Regul. 2016 Jan;60:29-35. doi: 10.1016/j.jbior.2015.10.004. Epub 2015 Oct 28. Adv Biol Regul. 2016. PMID: 26554303 Free PMC article. Review.
-
The novel poly(A) polymerase Star-PAP is a signal-regulated switch at the 3'-end of mRNAs.Adv Biol Regul. 2013 Jan;53(1):64-76. doi: 10.1016/j.jbior.2012.10.004. Epub 2012 Oct 13. Adv Biol Regul. 2013. PMID: 23306079 Free PMC article. Review.
-
Coordinated activation of the nuclear ubiquitin ligase Cul3-SPOP by the generation of phosphatidylinositol 5-phosphate.J Biol Chem. 2008 Mar 28;283(13):8678-86. doi: 10.1074/jbc.M710222200. Epub 2008 Jan 24. J Biol Chem. 2008. PMID: 18218622
-
A PtdIns4,5P2-regulated nuclear poly(A) polymerase controls expression of select mRNAs.Nature. 2008 Feb 21;451(7181):1013-7. doi: 10.1038/nature06666. Nature. 2008. PMID: 18288197
-
Diacylglycerol kinase-theta is localized in the speckle domains of the nucleus.Exp Cell Res. 2003 Jul 1;287(1):143-54. doi: 10.1016/s0014-4827(03)00115-0. Exp Cell Res. 2003. PMID: 12799190
Cited by
-
Phosphoinositides: tiny lipids with giant impact on cell regulation.Physiol Rev. 2013 Jul;93(3):1019-137. doi: 10.1152/physrev.00028.2012. Physiol Rev. 2013. PMID: 23899561 Free PMC article. Review.
-
Signaling through non-membrane nuclear phosphoinositide binding proteins in human health and disease.J Lipid Res. 2019 Feb;60(2):299-311. doi: 10.1194/jlr.R088518. Epub 2018 Sep 10. J Lipid Res. 2019. PMID: 30201631 Free PMC article. Review.
-
Phospholipase D2 is localized to the rims of the Golgi apparatus in mammalian cells.Mol Biol Cell. 2002 Nov;13(11):3930-42. doi: 10.1091/mbc.02-04-0059. Mol Biol Cell. 2002. PMID: 12429836 Free PMC article.
-
SF-1 Induces Nuclear PIP2.Biomolecules. 2023 Oct 12;13(10):1509. doi: 10.3390/biom13101509. Biomolecules. 2023. PMID: 37892191 Free PMC article.
-
T cells transduce T-cell receptor signal strength by generating different phosphatidylinositols.J Biol Chem. 2019 Mar 29;294(13):4793-4805. doi: 10.1074/jbc.RA118.006524. Epub 2019 Jan 28. J Biol Chem. 2019. PMID: 30692200 Free PMC article.
References
-
- Asano M, Tamiya-Koizumi K, Homma Y, Takenawa T, Nimura Y, Kojima K, Yoshida S. Purification and characterization of nuclear phospholipase C specific for phosphoinositides. J Biol Chem. 1994;269:12360–12366. - PubMed
-
- Balboa MA, Insel PA. Nuclear phospholipase D in Madin-Darby canine kidney cells. Guanosine 5′-O-(thiotriphosphate)-stimulated activation is mediated by RhoA and is downstream of protein kinase C. J Biol Chem. 1995;270:29843–29847. - PubMed
-
- Bazenet CE, Ruano AR, Brockman JL, Anderson RA. The human erythrocyte contains two forms of phosphatidylinositol-4-phosphate 5-kinase which are differentially active toward membranes. J Biol Chem. 1990;265:18012–18022. - PubMed
-
- Boronenkov IV, Anderson RA. The sequence of phosphatidylinositol-4-phosphate 5-kinase defines a novel family of lipid kinases. J Biol Chem. 1995;270:2881–2884. - PubMed
-
- Brockman JL, Anderson RA. Casein kinase I is regulated by phosphatidylinositol 4,5-bisphosphate in native membranes. J Biol Chem. 1991;266:2508–2512. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

